- LOGIN
- MemberShip
- 2025-12-23 06:05:25
- K-similars set out to enter the global market
- by Son, Hyung-Min | translator Kim, Jung-Ju | 2023-12-29 05:40:01
Domestic companies are now ready to launch homegrown biosimilars into the global market next year.
According to industry sources on the 28th, Celltrion, Samsung Bioepis, and Dong-A ST have completed Phase III clinical trials of its biosimilars and applied for approval from overseas regulators.
The companies have successfully developed biosimilars for their global blockbusters such as Stelara and Prolia and are seeking approval next year.
Celltrion expects to receive approval for 3 biosimilars next year Celltrion has the largest biosimilar pipeline among domestic companies.
To date, the company has 12 pipelines, with six products on the market that include the COVID-19 drug Regkirona, and four more that completed Phase III trials and are on track for marketing authorizations.
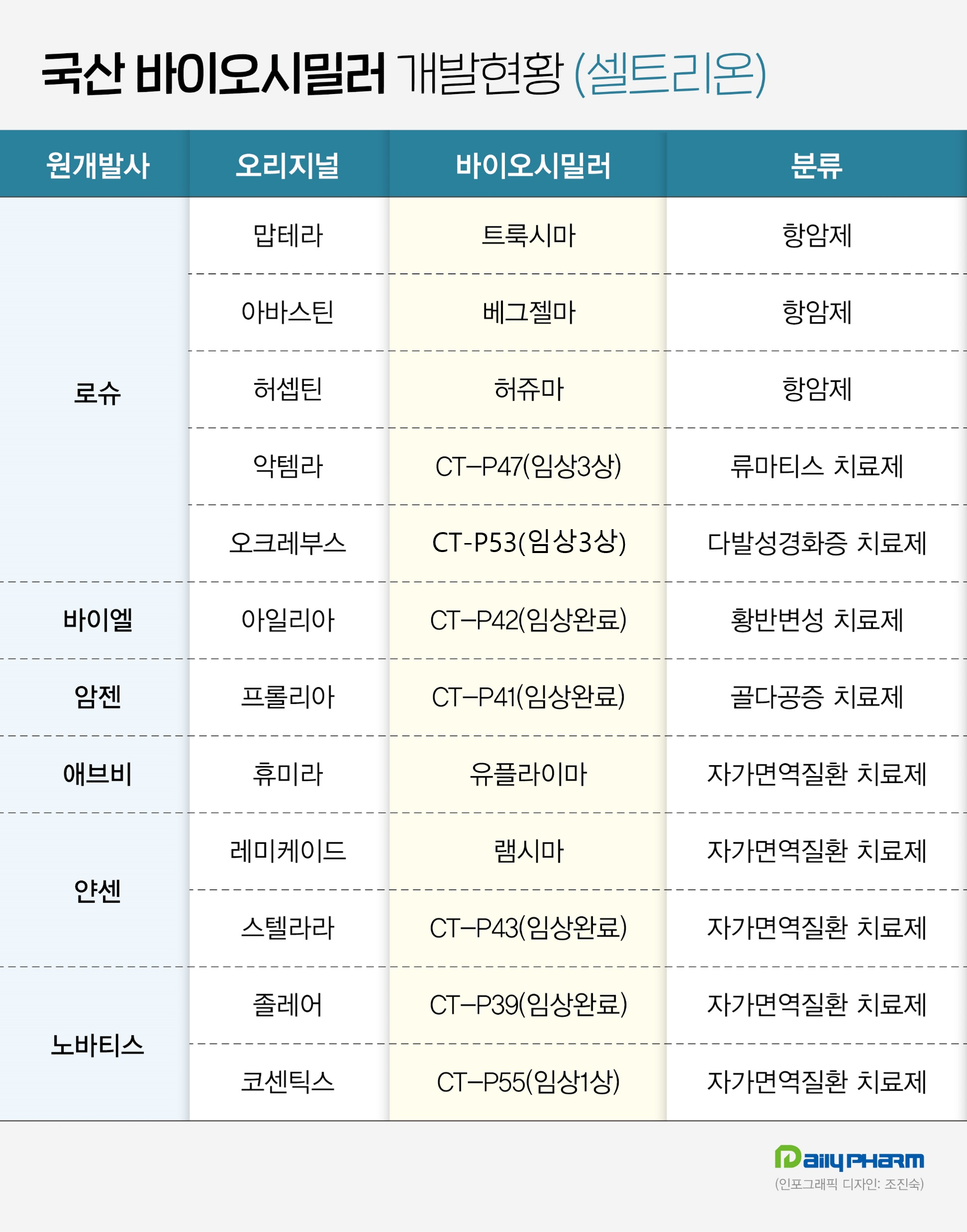
In Phase III trials, the four candidates demonstrated non-inferiority to their respective original drugs.
Based on the global Phase III results of CT-P41 this month, Celltrion filed for marketing authorization of CT-P41 for all of Prolia's U.S.
indications.
Prolia is an Amgen-developed osteoporosis treatment with multiple indications, including giant cell tumor of bone and bone loss.
After the U.S., Celltrion plans to file for approval in other key global markets, including Europe.
This month, Celltrion also completed the filing of a marketing authorization application for CT-P39 in Canada.
The original CT-P39 product, Xolair, is an antibody biologic developed by Novartis for the treatment of allergic asthma, chronic rhinosinusitis with nasal polyposis, and chronic spontaneous urticaria.
Xolair is a blockbuster product that generated approximately $5 trillion in global sales last year.
Celltrion is also nearing commercialization of its Stelara biosimilar CT-P43.
To date, it has filed for approval in the U.S., Europe, South Korea, and Australia.
Stelara is an interleukin (IL)-12 and 23 inhibitor developed by Johnson & Johnson’s subsidiary Janssen and is used to treat autoimmune diseases such as plaque psoriasis, psoriatic arthritis, Crohn's disease, and ulcerative colitis.
The global Stelara market was valued at approximately $23 trillion last year.
Celltrion finalized an agreement with Johnson & Johnson last year that will allow the drug to be sold in the US starting Feb.
22 next year.
Celltrion also recently completed a Phase III clinical trial for its Eylea biosimilar CT-P42 and recently filed for its marketing authorization in the U.S.
and Europe.
Ayla is a blockbuster macular degeneration treatment developed by Bayer and Regeneron that generated approximately $12 trillion in global sales last year.
With such a diverse pipeline, Celltrion aims to have a portfolio of 22 biosimilars by 2030.
Samsung Bioepis nears approval of its Prolia biosimilar next year Samsung Bioepis has successfully commercialized five biosimilars in overseas markets in collaboration with its global marketing partners Biogen and Organon.
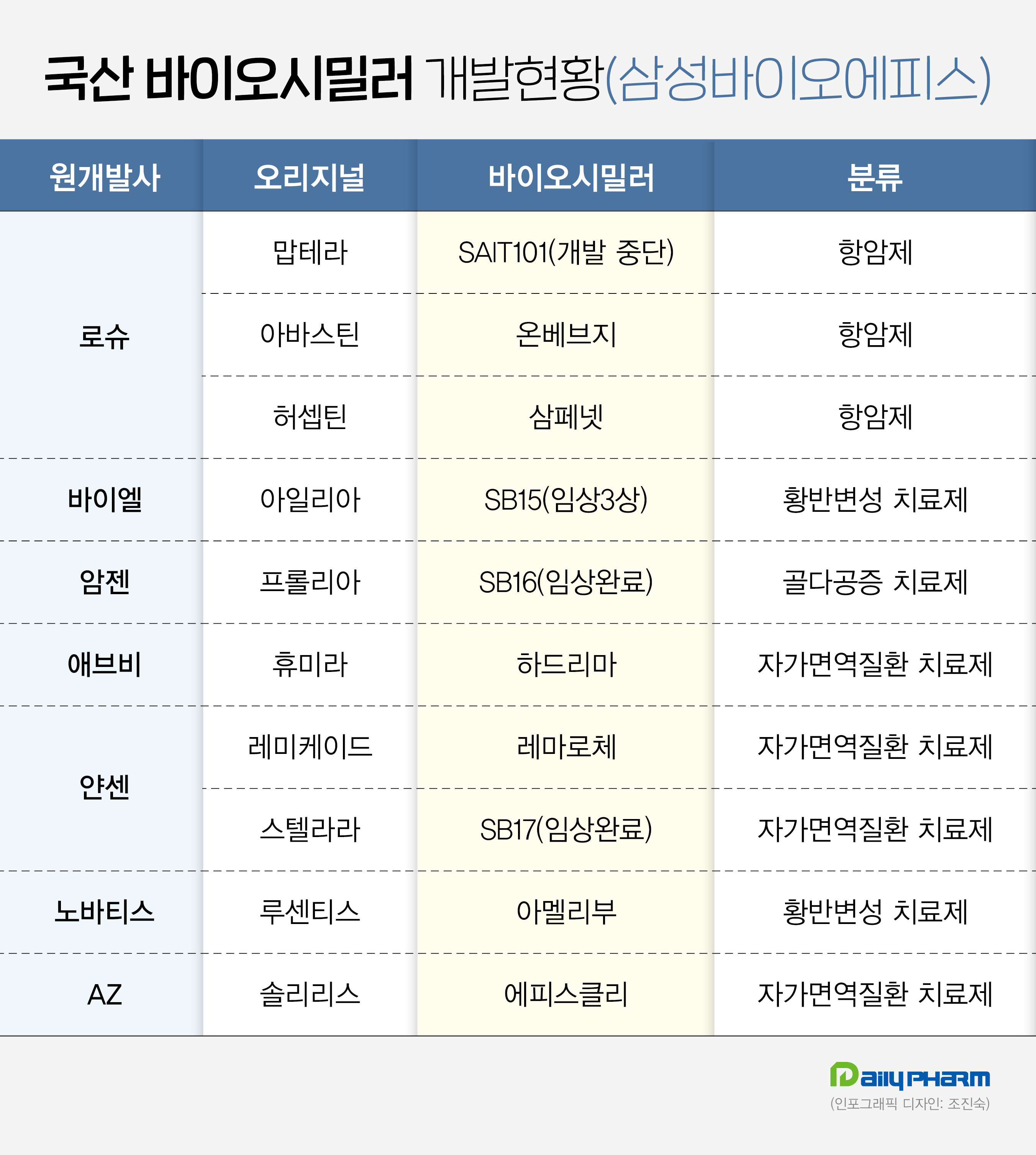
In a Phase III clinical trial conducted on postmenopausal osteoporosis patients, SB16 demonstrated equivalent efficacy to the original drug in terms of change from baseline in lumbar spine and bone mineral density at 12 months after administration.
Also, Samsung Bioepis has completed clinical trials of SB15, its Eylea biosimilar, and is pursuing approval procedures in the U.S.
and Europe.
Samsung Bioepis conducted a Phase III clinical trial involving 449 patients with wet age-related macular degeneration (nAMD) in 10 countries, including the United States and Korea.
In the trial, Samsung Bioepis evaluated patients' best-corrected visual acuity (BCVA) up to 56 weeks of treatment with SB15 and found comparable BCVA improvements compared to the original drug.
Samsung Bioepis plans to accelerate its expansion in the European market by emphasizing that its biosimilar, SB15, is interchangeable with the original drug.
In addition, in May last year, Samsung Bioepis obtained domestic marketing authorization for Lucentis' biosimilar Amelivu, which was approved in the U.S.
and Europe in the second half of 2021.
Lucentis, a macular degeneration treatment developed by Novartis, is a blockbuster drug that generated global sales of around KRW 4 trillion in 2020.
Dong-A ST and Sam Chung Dang Pharm enters the biosimilar competition…submits an application to regulatory authorities Dong-A ST’s Stellara biosimilar DBM-3115 is expected to be approved in Europe next year.
The company completed submitting its application to the European Medicines Agency (EMA) in June after demonstrating therapeutic equivalence between its DBM-3115 and Stellara in a global Phase III trial.
The company is also preparing for U.S.
approval next year.
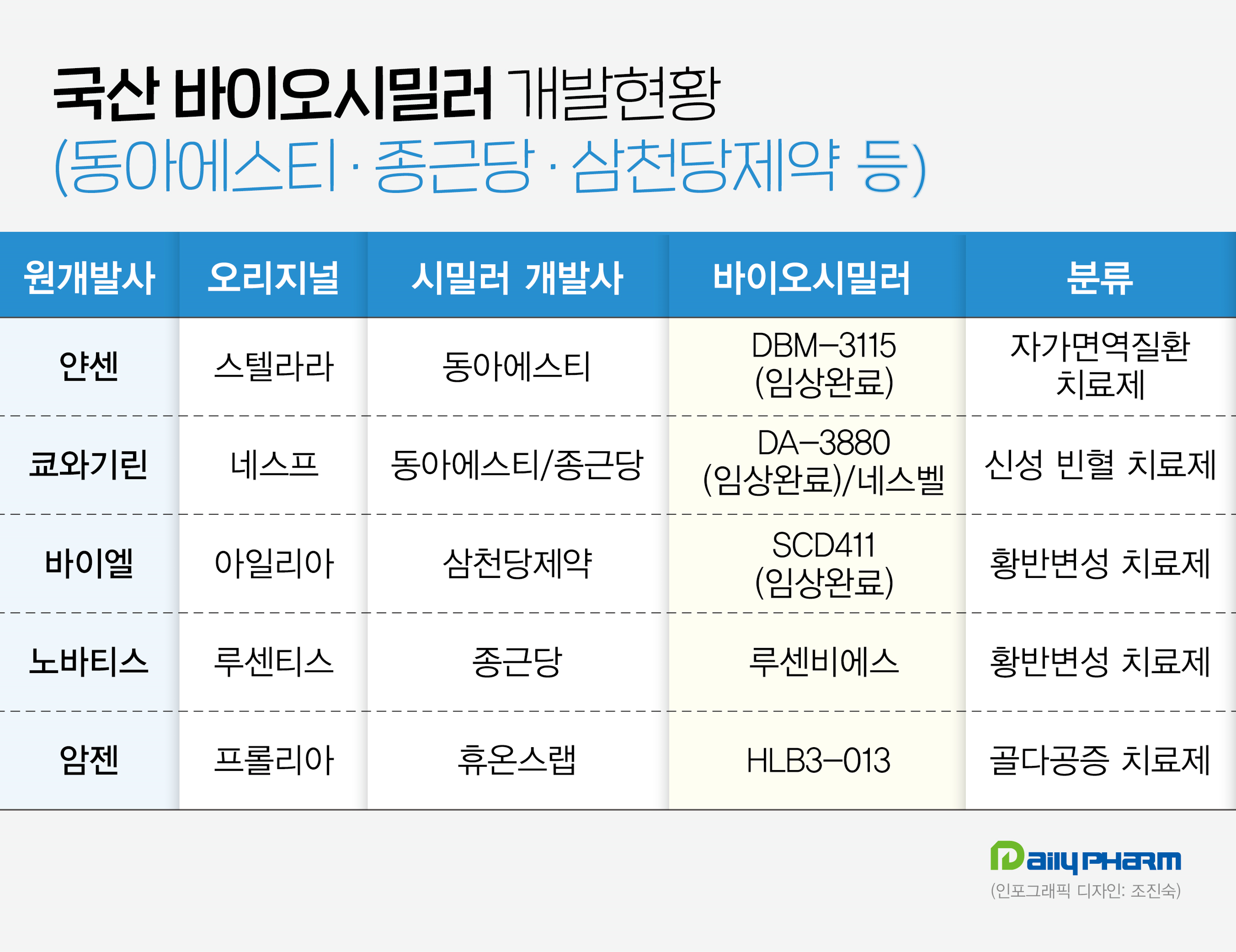
Sam Chun Dang is also preparing for its approval in the United States and Europe.
The application was made based on the results of the global Phase III clinical trial on SCD411.
In a clinical trial involving 576 patients with macular degeneration, SCD411 demonstrated equivalence to Eylea.
In addition, Huon's Global subsidiary, Huons Lab, is also accelerating the development of its Prolia biosimilar, HLB3-013.
In February, the company announced that it had confirmed equivalence in nonclinical animal efficacy studies compared to the original product.
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.










