- LOGIN
- MemberShip
- 2025-12-23 06:04:29
- Recent tech export deals boast record-high upfront payments
- by Chon, Seung-Hyun | translator Kim, Jung-Ju | 2024-01-09 05:49:34
Since the end of last year, Orum Therapeutics, Chong Kun Dang Pharmaceutical (CKD Pharm), LegoChem Biosciences, and others have successfully secured large-scale technology transfer agreement with an upfront payment of 100 billion won.
Out-licensing contracts with upfront payment scale over 10% of the total contract value have been on the rise due to the high value of technology exports for new drugs.
According to the industry on the 9th, LG Chem signed a technology transfer agreement with Rhythm Pharmaceuticals on the 5th for a rare obesity drug candidate LB54640.
Under the agreement, Rhythm Pharmaceuticals will acquire the global development and sales rights of LB54640.
LB54640 is the world's first oral MC4R agonist, and the results of its Phase 1 clinical trials have shown a dose-dependent trend in weight loss and safety.
LB54640 successfully completed Phase 1 clinical trials and commenced Phase 2 clinical trials in October.
Rhythm Pharmaceuticals will acquire the rights to LB54640 and continue its development.
Under the agreement, the price for technology transfer amounts up to $305 million (about 400 billion won), including the upfront payment of $100 million (approx.
130 billion won).
The upfront payment of $100 million is the third-largest in technology transfer agreement for a new drug signed by a Korean pharmaceutical firm.
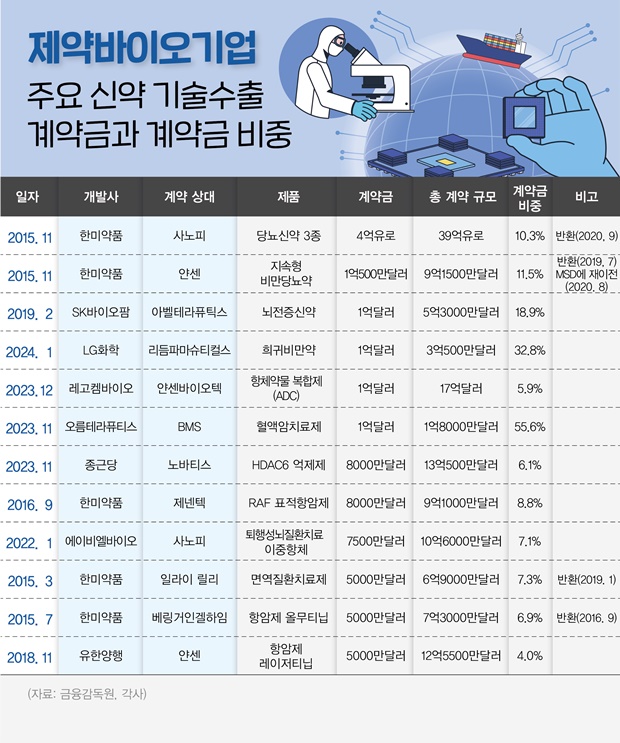
(Source: Financial Supervisory Service, Pharma companies) The highest record of upfront payment for technology transfer agreements is held by Hanmi Pharm.
In Nov.
2015, Hanmi Pharm signed a technology transfer agreement for three new diabetes drugs with Sanofi, which initially involved an upfront payment of approximately EUR 400 million.
The contract was later revised, reducing the upfront payment to EUR 204 million, but it still stands as the highest upfront payment in such agreements.
Hanmi Pharm also holds the record for the second-highest upfront payment with $105 million in an agreement with Johnson & Johnson’s Janssen for its obesity drug in 2015.
The upfront payment of $100 million in a technology transfer agreement between SK Biopharmaceuticals and Arvelle Therapeutics for cenobamate, an anti-epileptic drug, ranks third to date.
Recently, LG Chem also secured the third in rank with an upfront payment of $100 million in a technology transfer agreement for a new drug, LB54640.
The technology export contracts that were finalized since the end of last year have ranked among the top contracts in terms of upfront payments.
Orum Therapeutics signed a technology transfer agreement with BMS for the new candidate product ORM-6151 in Nov.
2023.
The total contract value was set at a maximum of $180 million, which included the upfront payment of $100 million.
ORM-6151 is a candidate product developed utilizing the antibody-based protein degradation platform of Orum Therapeutics.
It has received approval for Phase 1 clinical trials from the U.S.
Food and Drug Administration (FDA) as a potential treatment for myeloid leukemia and high-risk myelodysplastic syndromes.
LegoChem Bio also finalized a technology transfer agreement with an upfront payment of $100 million.
In December of the previous year, LegoChem Bio signed a technology transfer agreement with Janssen Biotech for the development and commercialization of "LCB84." The contract included an upfront payment of $100 million (approx.
130 billion won), an additional $200 million (approx.
260 billion won) payment for the exclusive development rights, and further milestone payments linked to development, approval, and commercialization, amounting to a total of up to $1.7 billion (approximately 2.24 trillion won).
LCB84 is an Antibody-Drug Conjugate (ADC) drug that combines LegoChem Bio's next-generation ADC platform technology with the Trop2 antibody technology acquired from Mediterranea Theranostic.
CKD Pharm signed a technology transfer agreement with Novartis for the new drug candidate CKD-510 in Nov.
2023.
The non-refundable upfront payment for this agreement was $80 million, ranking seventh to date.
Including milestones of $1.225 billion based on development and approval stages, the total contract size amounts up to $1.35 billion.
CKD-510 is a new drug candidate developed by CKD Pharm, utilizing a highly selective non-hydroxamic acid (NHA) platform technology-based HDAC6 inhibitor.
Recently, in the technology transfer agreements of pharmaceutical and biotech firms, upfront payments have been a substantial component of the total contract value.
LG Chem's LB54640 technology transfer agreement had an upfront payment that accounted for 32.8% of the total contract value, which is a significantly higher proportion than the usual practice where upfront payments typically do not exceed 10% of the total contract value.
LG Chem explained that this high value reflects the positive assessment of LB54640's growth potential by their technology transfer partner.
Orum Therapeutics received an upfront payment of $100 million, which accounted for 55.6% of the total contract value in their technology transfer agreement with BMS last year.
However, Orum Therapeutics' technology transfer involved the transfer of a new drug candidate, which contributed to the larger upfront payment.
Typically, pharmaceutical companies receive milestone payments based on the progress of development in technology transfer agreements.
In this case, Orum Therapeutics chose to transfer rights and received a larger upfront payment.
Hanmi Pharmaceutical's technology transfer agreement with Sanofi for three diabetes drugs, holding the record for the highest upfront payment, had an upfront payment representing 10.3% of the total contract value.
However, Hanmi Pharmaceutical and Sanofi's technology transfer agreement saw a reduction in contract size through a revised contract, with the upfront payment ratio decreasing to 7.2%.
In 2015, Hanmi Pharmaceutical's technology transfer to Janssen for an obesity and diabetes treatment recorded a high upfront payment ratio of 11.5%.
Despite being in the early stages of development, the technology transfer partner assessed the candidate substance with high value.
In 2019, SK Biopharmaceuticals entered into a technology transfer agreement with Arvelle Therapeutics for cenobamate, and the upfront payment representing 18.9% of the total contract value.
At that time, cenobamate was already in the process of FDA review in the United States, indicating a high likelihood of commercialization, which contributed to the substantial upfront payment in the agreement.
The proportion of upfront payments to the total contract value for CKD Pharm and LegoChem Biosciences accounted for 6.1% and 5.9%, respectively.
The analysis suggests that the high upfront payments were made because the partnering companies have made positive assessments of new drugs, even though they are in the early stages of clinical trials.
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.










