- LOGIN
- MemberShip
- 2025-12-23 06:05:38
- SGLT2i+DPP4i combos' performance falls short of expectations
- by Kim, Jin-Gu | translator Kim, Jung-Ju | 2024-01-23 06:02:23
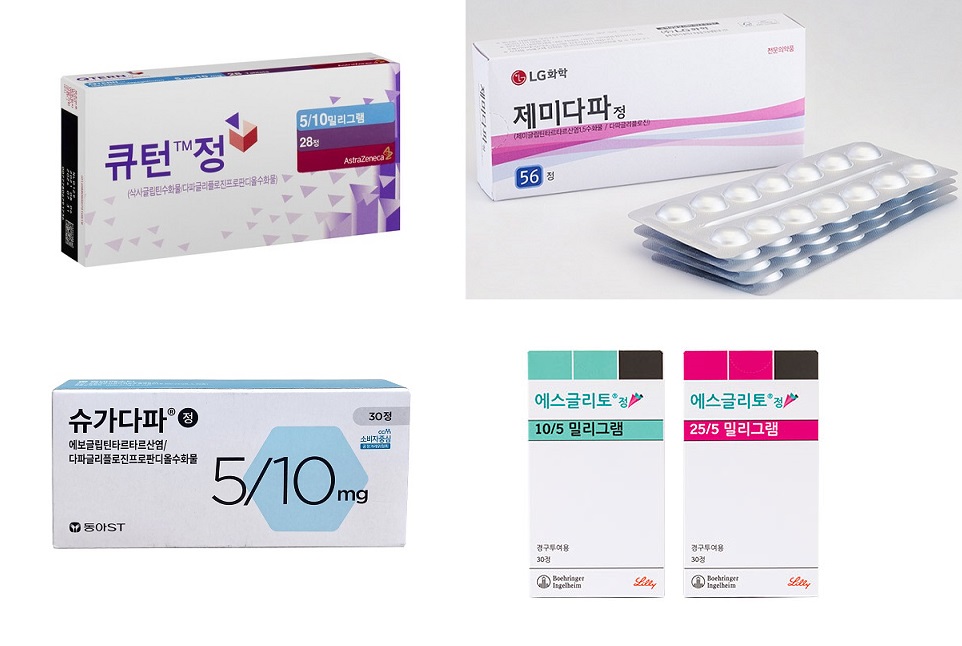
Of the major products that have been on the market since May, only 3 generated more than KRW 2 billion in prescriptions by the end of the year.
Of the more than 30 dapagliflozin+sitagliptin combination products released since September, most prescriptions sold less than KRW 100 million by the end of the year.
In the field, prescribers are noting how the extended reimbursement is applied to the 3 drug combinations of SGLT2i+DPP4i+metformin.
Therefore, rather than adding metformin to the newly launched SGTL2i+DPP4i two-drug combination drugs, prescribers have been mainly prescribing the combination by adding a single SGLT2i agent to existing DPP4i+metformin combination drugs.
SGLT2i+DPP4i combo mkt size KRW 8.8 bil… Esglito>Zemidapa>Qtern According to the pharmaceutical industry on the 23rd, the outpatient prescription market for SGLT2i+DPP4i combination was KRW 8.8 billion last year.
Behringer Ingelheim's Esglito (empagliflozin+linagliptin)’ has shown the highest prescription performance, generating KRW 2.7 billion in prescription sales in the 8 months following its reimbursement listing in May last year.
This was followed by LG Chem's Zemidapa (dapagliflozin+Zemiglo) and AstraZeneca's Qtern (dapagliflozin+saxagliptin), each of which recorded KRW 2.1 billion.
Qtern is being sold by Ildong Pharmaceutical in Korea.
The rest of the combination drugs have all posted less than KRW 1 billion in sales.
Chng Kun Dang’s ‘Exiglu-S Tab (dapagliflozin+sitagliptin) posted KRW 600 million, Dong-A ST’s Sugadapa (dapagliflozin+evogliptin) posted KRW 500 million, and AstraZeneca's Sidapvia (dapagliflozin+sitagliptin) for KRW 200 million.
Of these, all products other than Sidapvia, have been sold in earnest since their reimbursement listing in May last year.
Sidapvia was launched in September last year after the patent expiry of Januvia (sitagliptin).
The drug is manufactured and supplied by SK Chemicals in Korea.
Other dapagliflozin+sitagliptin combinations launched alongside Sidapvia have accumulated less than KRW 100 million in prescriptions from September until the end of the year.
Industry expresses performance is 'below expectations'...Different response from when the 96 companies were approved The market was formed after reimbursement was extended to combination therapy in Korea for diabetes in April last year.
At the time, the government extended reimbursement to the SGLT2i+DPP4i+metformin combination.
In May, the SGLT2i+DPP4i combination drugs were approved The pharmaceutical industry had initially predicted that demand would rise for combination products, especially those that combined SGLT2i and DPP4i since it was the first time the combined use of SGLT2i and DPP4i was reimbursed in Korea.
In fact, the pharmaceutical industry had shown great attention, with 96 companies receiving approval for the 2-drug combo around the period of the reimbursement expansion.
In April, the patent for the flagship SGLT2i class drug Forxiga (dapagliflozin) expired.
The companies that owned original DPP4i drugs rushed to launch combination products that contained dapagliflozin.
In September, the patent for Januvia (sitagliptin), the flagship DPP4i class drug, also expired.
The expiration of the patents for the top drugs in each class led to a flurry of launches of dapagliflozin+sitagliptin combinations.
But now last year's outpatient prescription performance results are out, and the industry consensus is that the performance of the 2-drug combinations is a disappointment.
Even when considering that it was the first year of sales, it was not up to expectations.
When taking the dapagliflozin+sitagliptin two-drug combination as an example, 34 companies have launched their product since September, but their combined prescription sales amounted to only KRW 1.4 billion.
The average prescription per company is less than KRW 50 million.
Combination drugs that used original drugs fared similarly.
Taking Esgliteo, the highest-selling drug last year that posted KRW 2.7 billion as an example, its components, the single-agent drugs ‘Jadiance (empagliflozin)’ and ‘Trajenta (linagliptin),’ generated prescription sales of KRW 58.1 billion and KRW 61.3 billion, respectively, last year.
"Prescribing the more familiar DPP4i+metformin combination"...New combinations at a crossroads The industry has raised various interpretations on the lower-than-expected performance of the drugs.
First of all, one analysis was that the new combination drugs were not well received in the field.
The government's diabetes benefit extension was for the three-drug combination of SGLT2i+DPP4i+metformin.
The SGLT2i-DPP4i two-drug combination by itself is not covered.
As a result, prescribers were left to choose one of three options: SGLT2i+DPP4i combo and metformin, SGLT2i+metformin combo and DPP4i, or SGLT2i and DPP4i and metformin.
The problem is that prescribers were relatively more familiar with the SGLT2i+metformin or DPP4i+metformin combinations that were already on the market, compared with the more newly introduced SGLT2i+DPP4i combinations.
"To use the newly approved SGLT2i+DPP4i combinations, patients would need to change all of their existing medications, whereas DPP4i+metformin combinations require patients to add a single SGLT2i to their existing medications, so there is less patient resistance," explained one endocrinologist.
"Also, the combination drugs are not very competitive price-wise when compared with prescribing each drug separately," he added.
In the case of the dapagliflozin plus sitagliptin combination, supply issues with sitagliptin also played a role.
In the case of sitagliptin, one contract manufacturer manufactures products for 10 or more companies.
The production capacity of the contract manufacturers has reportedly become overwhelmed, manufacturing the ingredients for so many companies.
Some companies are also reportedly having difficulty producing products for companies due to difficulty procuring raw materials that need to be imported from India.
However, there is also industry opinion that it is too early to tell whether SGLT2i+DPP4i combination products will be successful in the market.
As there is still a possibility that the sitagliptin contract manufacturers will be able to stabilize their supply, and as related products are in the early stages of their launch, there remains enough potential for future market expansion.
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.










