- LOGIN
- MemberShip
- 2025-12-23 06:05:38
- Vemlidy dominates stagnant 300 bil. won hepatitis B market
- by Son, Hyung-Min | translator Kim, Jung-Ju | 2024-01-29 06:05:17
The market for hepatitis B treatment, worth 300 billion won annually, is currently experiencing a slowdown.
While Gilead Sciences Korea’s Vemlidy is expanding its market share, Viread and Baraclude are facing a decline.
In the generics market, Dong-A ST is leading the market.
Prescription sales for Vemlidy has increased by 26% compared to last year, while original drugs have shown poor performance According to the drug market research agency UBIST on the 26th, the market size for hospital outpatient prescriptions last year was 284.6 billion won, a 1.2% increase compared to 281.1 billion won in 2022.
Additionally, the market size for original drugs last year was 236 billion won, showing a slight increase from 229.2 billion won in 2022.
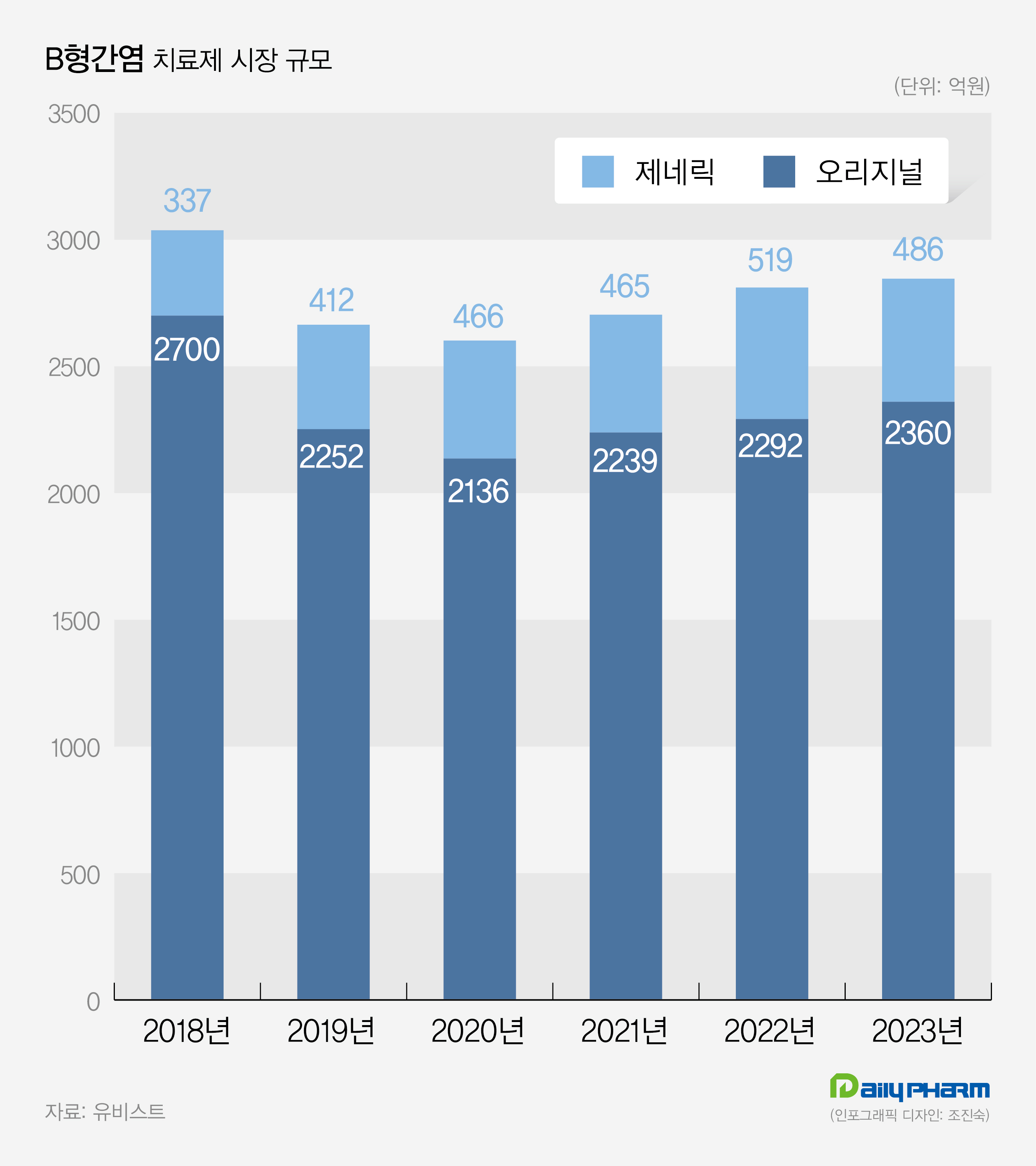
(Unit: KRW 100 million) Vemlidy is experiencing rapid growth in the market for treating hepatitis B.
The original drug's sales have significantly decreased since Viread’s drug pricing reduction in 2018, and Vemlidy seems to be filling the void.
Vemlidy entered the Korean market in 2017.
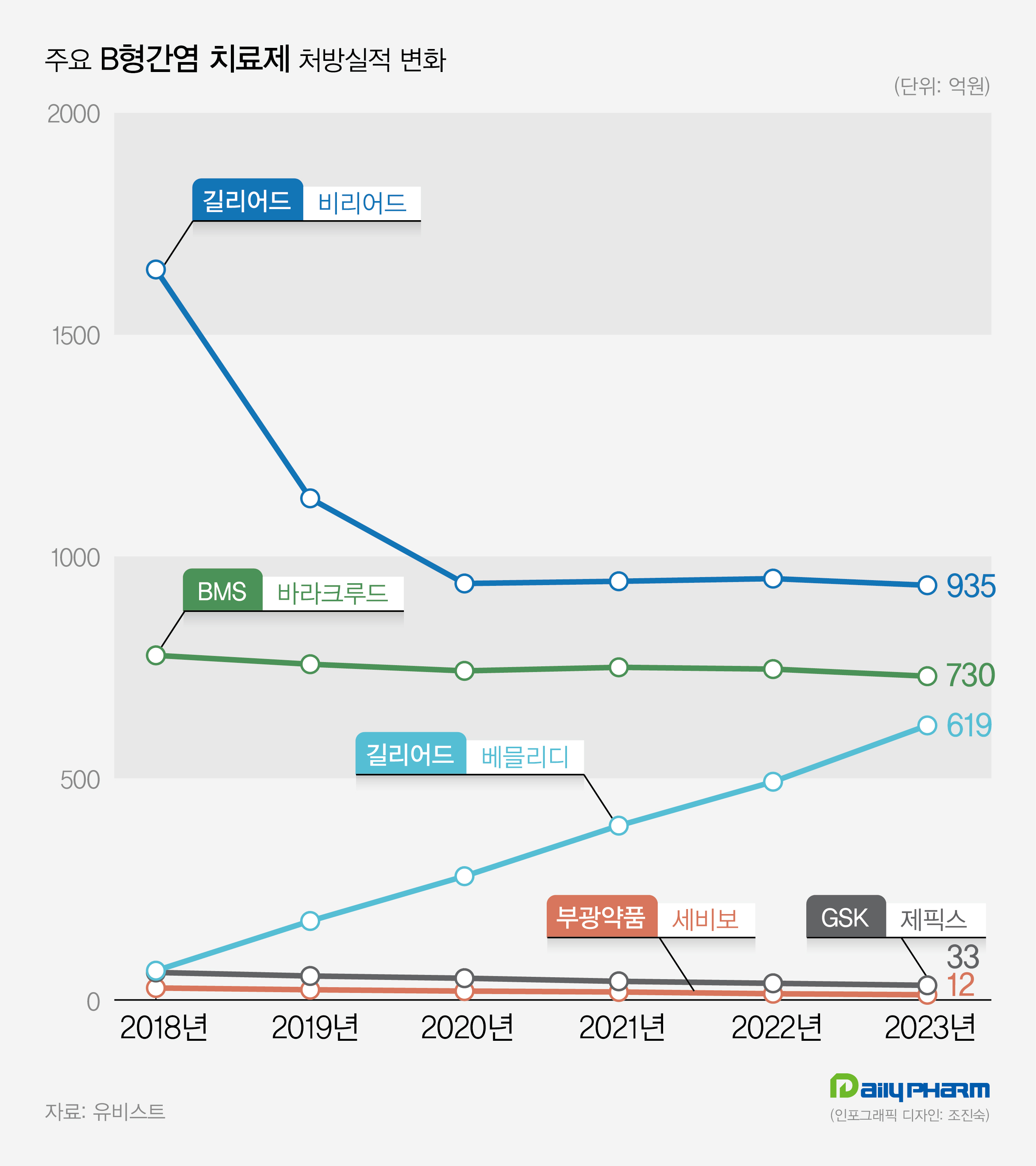
In 2019, its prescription sales exceeded 10 billion won, and the sales continued to grow, with 39.3 billion won in 2021 and 49.2 billion won in 2022.
The prescription sales of Vemlidy recorded 61.9 billion won last year, a 25.8% increase compared to the previous year.
Vemlidy, a new hepatitis B drug developed by Gilead Sciences, is a follow-up to Viread.
Despite its effectiveness in inhibiting the hepatitis B virus, Viread is known to cause side-effects such as kidney injury and low bone mineral density.
If any problem arises with kidney function after receiving treatments, reimbursement standards allow Vemlidy to be replaced by Baraclude.
Vemlidy overcomes Viread's adverse effects.
In clinical trials, Vemlidy treatment demonstrated effectiveness in the long-term treatment of patients and observed no side effects related to kidney injury or low bone mineral density.
Considering that hepatitis B cannot be cured, the safety of long-term administration is crucial to both patients and medical professionals.
Gilead Sciences is switching Viread, its first marketed hepatitis B drug, with Vemlidy.
(Unit: KRW 100 million) Prescription sales of Viread were 164.7 billion won in 2018, but they have been declining since then, reaching 113.1 billion won in 2019 and dropping to 90 billion won in 2020.
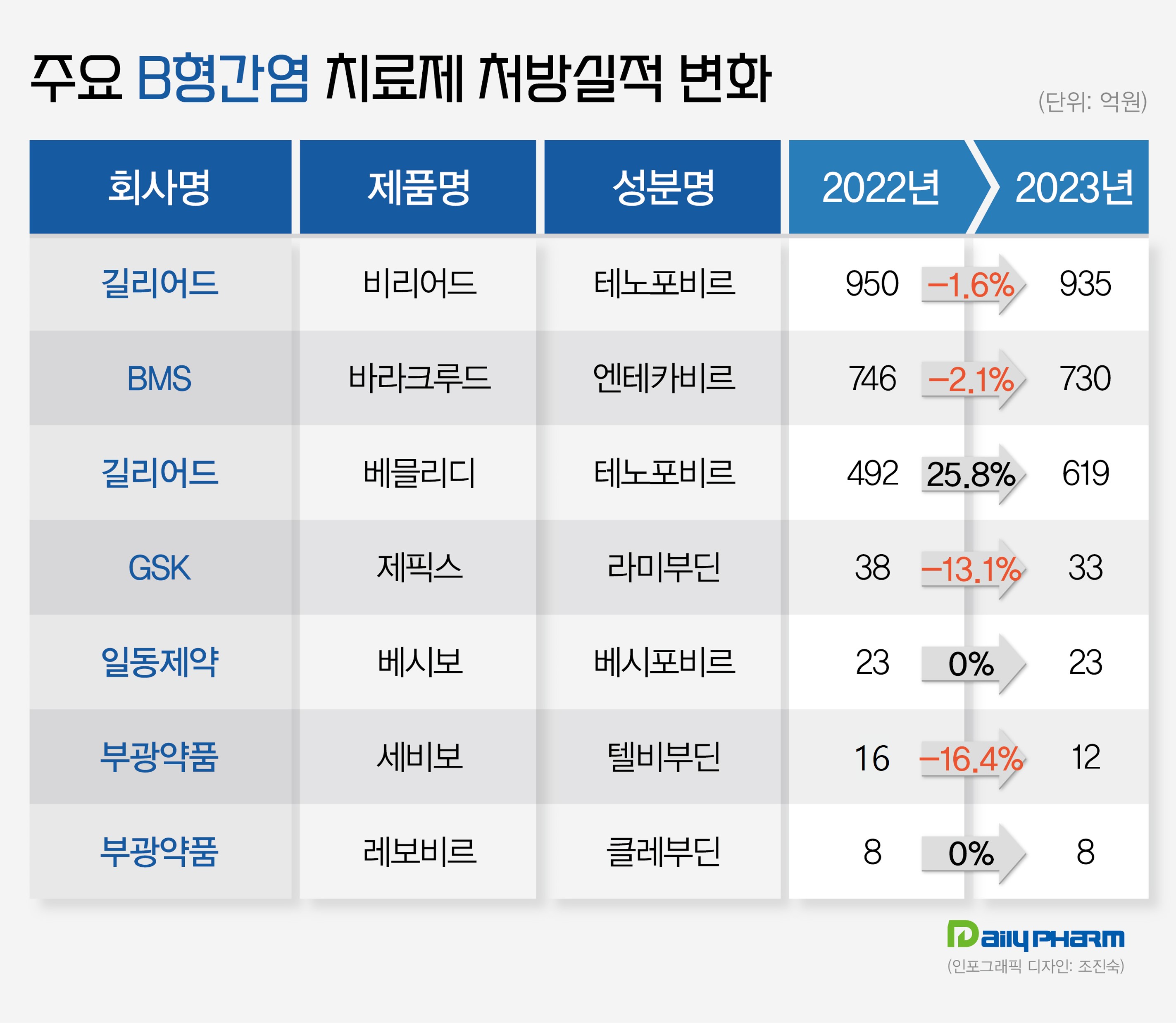
Last year’s prescription sales amounted to 93.5 billion won, a 1.6% decrease from the previous year.
Baraclude recorded prescription sales of 73 billion won last year, indicating a 2.1% decrease from 2022.
The analysis suggests that the decline in Baraclude sales can be attributed to a reduction in drug pricing.
BMS Pharmaceutical voluntarily reduced the price of Baraclude, which had expired patent, as part of a trade-off approach following the listing of its acute myeloid leukemia treatment Onureg and myelofibrosis treatment Inrebic for reimbursements.
Prescription sales for GSK's Zeffix decreased by 13.1% from 3.8 billion won in 2022 to 3.3 billion won last year.
GSK was unable to capture the market share left by Hepsera's withdrawal.
Hepsera, a new drug for hepatitis B developed by GSK, recorded over 10 billion won in prescription sales in 2018.
However, about a quarter of patients experienced resistance to the drug and experienced difficulties in maintaining treatment.
GSK withdrew the approval for Hepsera in 2022, leading to its withdrawal from the Korean market.
(Unit: KRW 100 million) Sales of treatments from domestic pharmaceutical companies were not significant.
Bukwang Pharm’s Sebivo topped prescription sales of 1.2 billion won, showing a 25% decrease from the previous year.
Sebivo, a Novartis drug introduced by Bukwang Pharmaceutical in 2019, saw a decline in prescription performance even after Bukwang took over sales.
Il Dong's Besivo maintained its performance by generating prescription sales of 2.3 billion won last year, holding steady compared to the previous year.
Besivo has not shown significant growth since its launch in 2017.
Levovir, Bukwang Pharm's in-house developed hepatitis B drug, maintained its performance from 2022 by achieving sales of 800 million won last year.
Levovir, the first domestically approved hepatitis B treatment, received approval in 2006.
However, its prescription sales have consistently declined, recording less than 1 billion won since 2021.
Generic drug growth slowed…Decreased sales of generic versions of Viread and Baraclude Last year, prescription sales for some generic drugs were reported to have decreased, just like certain original pharmaceuticals.
Generic drugs for hepatitis B recorded prescription sales of 48.6 billion won last year, showing a 6.3% decrease from 51.9 billion won in 2022.
During the same period, the market share of generics also slightly decreased from 23% to 21%.
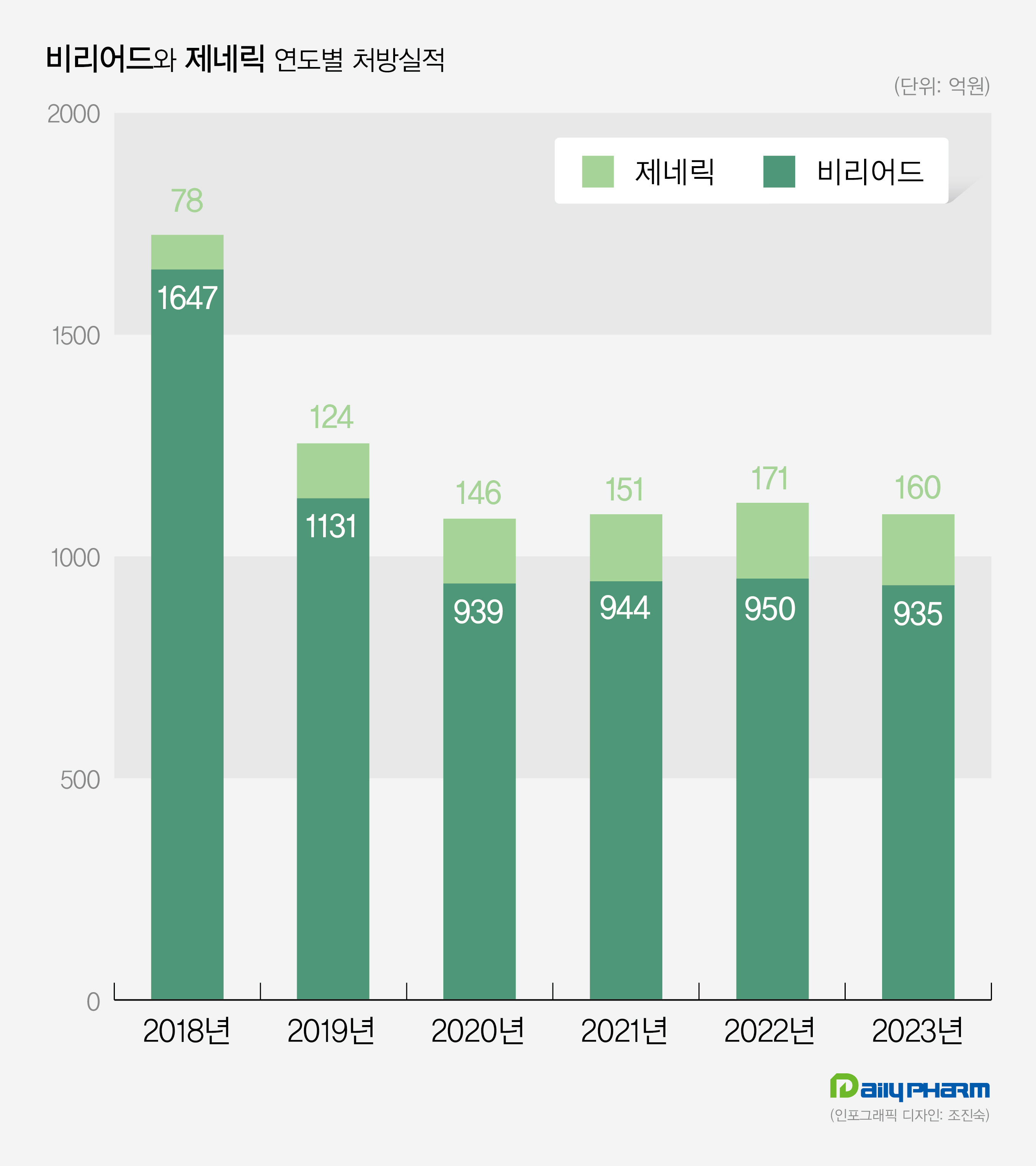
(Unit: KRW 100 million) Prescription sales for generic version of Viread decreased by 6.4% from 17.1 billion won in 2022 to 16 billion won last year.
Viread generic drug market has steadily increased prescription sales since surpassing 10 billion won in 2019, but its growth halted after five years.
Among them, Dong-A ST's Virreal, which holds the highest market share, experienced a 12.5% decrease in prescriptions, dropping from 3.2 billion won in 2022 to 2.8 billion won.
During the same period, Daewoong's Virehepa decreased by 11.8%, and Jeil Pharm’s Tecavir decreased by 15.2%.
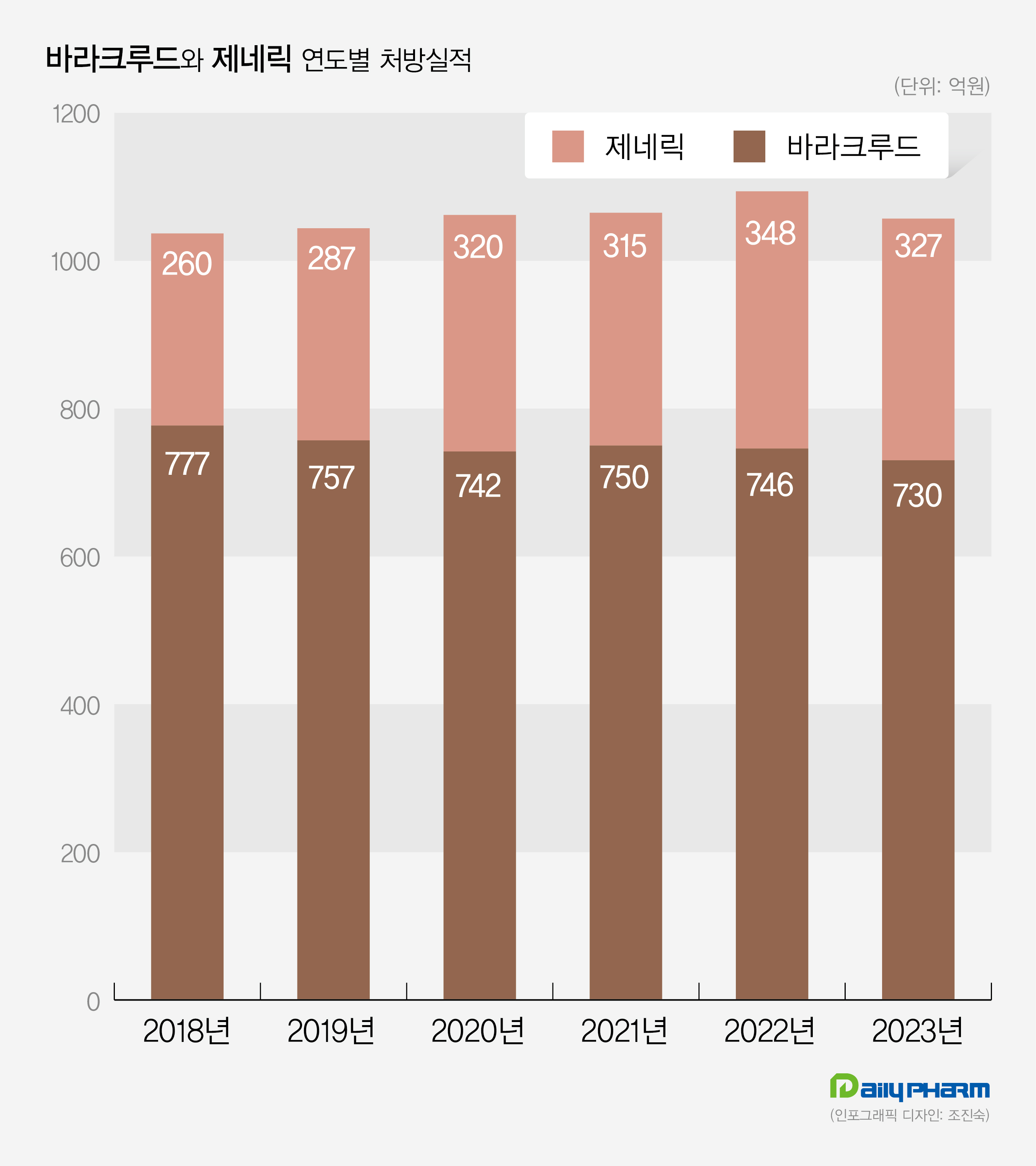
(Unit: KRW 100 million) Prescription sales for Baraclude's generic version were recorded at 33.3 billion won last year, a 4.5% decrease compared to 2022.
Although this market surpassed 30 billion won in prescription sales in 2020, it did not significantly increase its market share.
Dong-A ST's Baracle recorded prescription sales of 10.5 billion won last year, becoming the only generic drug to surpass the 10 billion won mark.
In this market, Dong-A ST held a 32% market share.
Bukwang Pharmaceutical's Bukwang entecavir recorded 3.2 billion won last year, a 29% decrease from 4.5 billion won in 2022.
During the same period, Daewoong Pharmaceutical's Baracross increased from 3.3 billion won to 3 billion won, indicating a 9% decrease.
The prescription sales for JW Pharmaceutical’s Entekhan decreased by 17%.
Generic drug developers' next target is Vemlidy.
Aside from the first generics like Dae Woong’s Vemliver, Dong-A ST’s Vemlia, and CKD Pharmaceutical’s Tenofobell-A, five other companies have received approval for generic versions of Vemlidy in the domestic market.
These generics were officially launched in the last quarter of the previous year, contributing to a combined prescription amount of 800 million won for the quarter.
It will be interesting to see how Vemlidy's growth might impact the expansion of the generic pharmaceuticals market.
Due to issues related to reimbursement cuts, switching between hepatitis B treatments has been challenging.
However, generic companies plan to increase their market share by offering lower drug prices.
Currently, Vemlidy costs 3,500 won, but domestic generic companies have entered the market with prices in the 2,400 won range, approximately 70% of Vemlidy's price.
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.










