- LOGIN
- MemberShip
- 2025-12-23 06:03:54
- SK Biopharm’s new drug tops 500 bln won in US sales
- by Kim, Jin-Gu | translator Kim, Jung-Ju | 2024-02-01 12:39:06

Previously, SK Biopharmaceuticals struggled with operating losses almost every quarter, except for occasional profits from technology exports.
However, the company has now turned to achieve profits, primarily attributed to the success of Cenobamate.
With its U.S.
direct sales system in place, SK Biopharmaceuticals anticipates a high gross profit margin increase of mid-90%.
SK Biopharm’s operating profit reached 15.2 billion won in Q4 last year, swinging to profitability after eight quarters of loss According to the pharmaceutical industry on the 30th, SK Biopharmaceuticals recorded 354.9 billion won in revenue last year, a 44% increase from the previous year at 246.2 billion won.
In the same period, the company’s operating loss reduced from 131.1 billion won to 37.1 billion won.
The company reduced its operating loss by 94 billion won year-on-year.
Notably, the company succeeded in turning black in almost eight quarters since Q4 2021.
The operating profit in Q4 was 15.2 billion won.
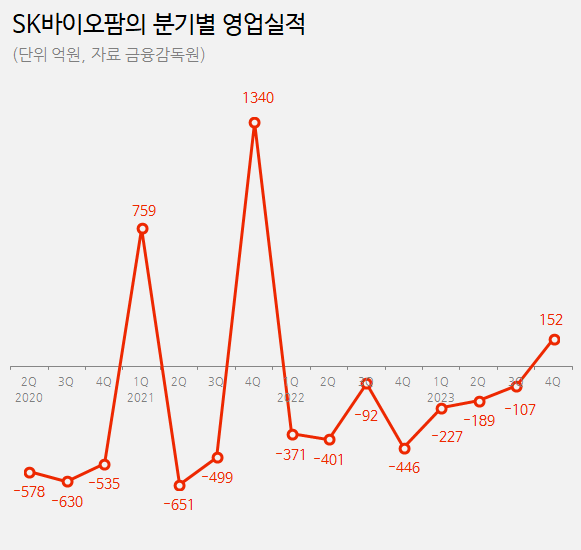
Except for occasional profits from technology exports in Q1 2021 (75.9 billion won surplus) and Q4 2021 (134 billion won surplus), the company continued to record operating losses.
In Q4 of last year, SK Biopharmaceuticals shifted to achieving operating profit, even without factoring in profits from technology exports.
The company attributes this success to the increased sales of Cenobamate in the United States.
Cenobamate’s sales in the U.S.
have seen a steep increase, accumulating a total of 530 billion won in sales Cenobamate’s sales in the United States were 270.7 billion won, a 60% increase year-on-year.
Cenobamate, which received approval from the United States Food and Drug Administration (FDA) in November 2019, was launched in May of the following year.
Since its launch, Cenobamate’s sales in the United States have experienced significant yearly growth, achieving 12.7 billion won in 2020, 78.2 billion won in 2021, and 169.2 billion won in 2022.
Since its launch, Cenobamate has generated a cumulative total of 530.8 billion won in sales.
The industry analysis predicts that if the current growth trend continues, sales in the U.S.
market will expand to approximately 400 billion won, with cumulative sales reaching 900 billion won by the end of this year.
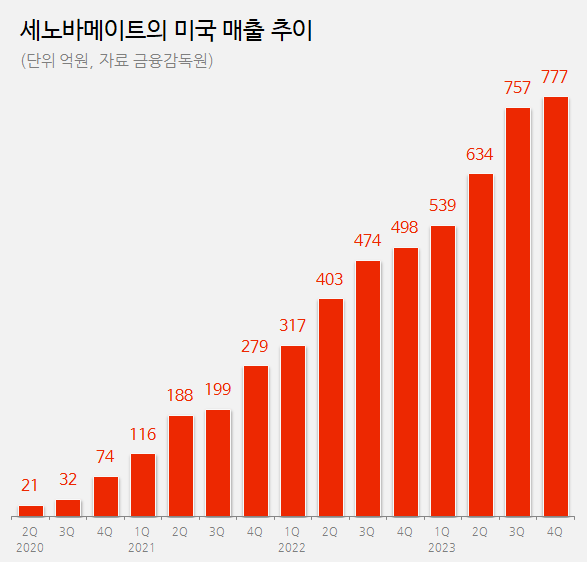
market (Unit: 100 mllion won, Source: Financial Supervisory Serivce). SK Biopharmaceuticals explained that Cenobamate’s new-to-brand prescriptions (NBRx) have steadily increased over the past year.
As of December last year, 44 months after its launch, Cenobamate recorded NBRx of 26,000, which is 2.2 times higher than that of the competing drug.
SK Biopharmaceuticals plans to increase the number of monthly Cenobamate prescriptions to 30,000.
Simultaneously, the company aims to become number one in recording prescriptions within the therapeutic area (TA) and accelerate its business growth.
SK Biopharm has established the U.S.
direct sales system and inventory adjustment in advance, anticipating “a high gross profit margin” SK Biopharmaceuticals expects that the steady growth in Cenobamate’s sales in the United States, which led to the operating profit achieved in Q4 of last year, will continue throughout this year.
The U.S.
direct sales system is the rooting force behind this positive outlook.
Before the launch of Cenobamate, SK Biopharmaceuticals established its U.S.
subsidiary, SK Life Science, and established an extensive distribution network nationwide.
They have also recruited specialized CNS sales professionals throughout the United States to directly promote Cenobamate.
SK Biopharmaceuticals explained that the company can achieve a high gross profit margin with this direct sales system.
Unlike selling through local partners, SK Biopharmaceuticals can achieve a high profit margin as it doesn't have to pass on approximately 30% of its revenue as commissions.
SK Biopharmaceuticals anticipates that its future gross profit margin will remain in the mid-90s range.
Another attributing factor for expecting a high gross profit margin is blocking any possibilities of operating losses in advance.
At the end of the previous year, SK Biopharmaceuticals adjusted their inventory to prevent excessive accumulation by local wholesalers.
This helps to reduce adverse impacts on operational performance resulting from inventory depletion.
SK Biopharm is planning to expand indications for Cenobamate and develop a new pipeline as part of its mid-to-ling-term strategy The mid-to-ling-term strategy focuses on expanding indications and age groups and developing a follow-up new product.
For Cenobamate's indications, SK Biopharmaceuticals plans to expand its approval from partial seizures to generalized seizures.
The expected timeline for approval of this expanded indication is between 2025 and 2026.
Additionally, SK Biopharmaceuticals intends to expand the age range of use from adults to pediatric and adolescent patients.
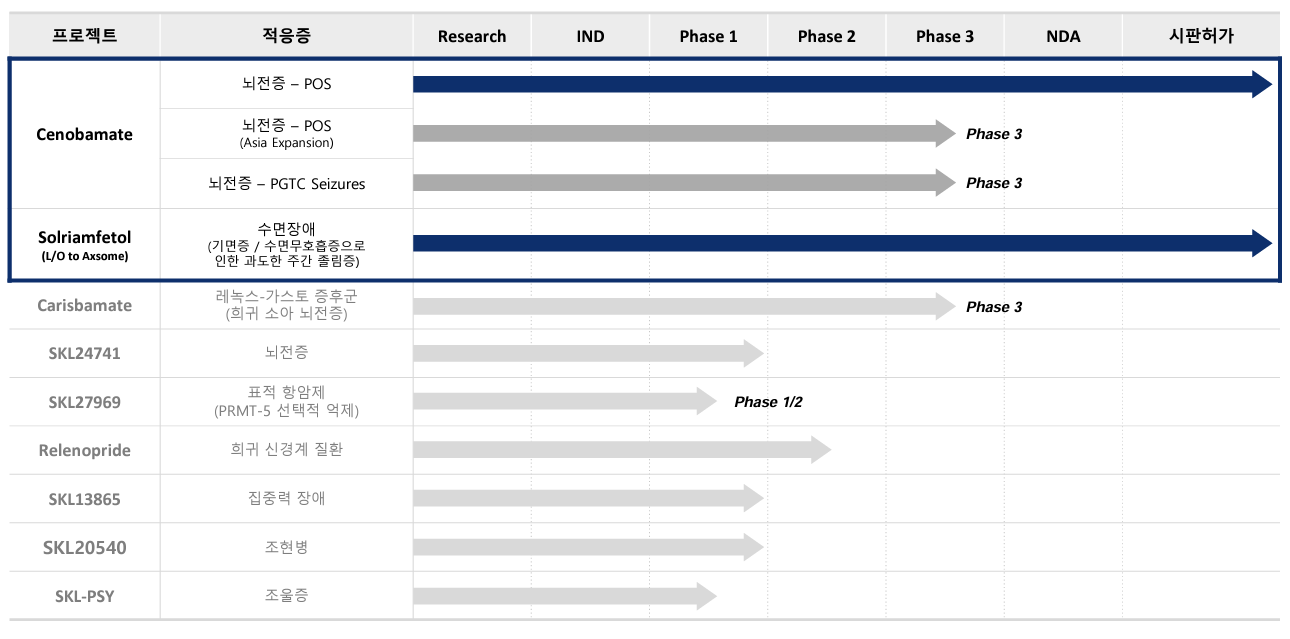
Last year, SK Biopharmaceuticals selected the development of radiopharmaceutical therapy (RPT), targeted protein degradation (TPD), and cell-gene therapy (CGT) as the ‘Three New Modality.’ Among these three, targeted protein degradation (TPD) is a potential candidate product.
Led by SK Life Science Labs, the company plans to provide information on the pipeline in the TPD area and the development schedule this year.
SK Life Science Labs owns a platform called ‘MOPED’ for discovering the molecular glue (MG).
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.










