- LOGIN
- MemberShip
- 2025-12-23 06:05:39
- Unstoppable sales growth of Entresto
- by Kim, Jin-Gu | translator Kim, Jung-Ju | 2024-02-13 06:18:36
Entresto has consistently achieved over 30% growth in sales each year since its release in October 2017, having passed six years.
Since its release, Entresto has undergone five price reductions to suppress the steep increase in prescription sales.
However, as Entresto’s simultaneous reimbursement range expanded, efforts to suppress sales were ineffective.
Prescription sales of Entresto exceeds 50 billion won.
The drug has shown a steep growth of more than 30% annually IQVIA, a drug market research agency, reported on the 8th that Entresto’s outpatient prescription sales reached 57.5 billion won last year, showing a 35% year-over-year (YoY) increase from 42.5 billion won in 2022.
Entresto is a new class of drugs called angiotensin receptor neprilysin inhibitor (ARNI), a combination of Valsartan, an angiotensin II receptor blocker (ARB) inhibitor, and Sacubitril, a neprilysin inhibitor.
After receiving reimbursement approval, Entresto was released in October 2017.
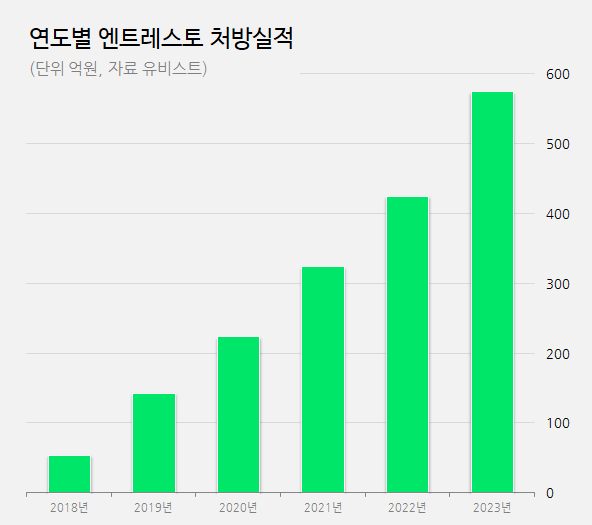
Entresto’s prescription sales grew from 5.5 billion won in 2018 to 14.3 billion won the following year, a 2.6-fold increase.
Despite the exacerbation of Covid-19 in 2020, Entresto continue to experience an upward trend in sales.
Annual prescription sales increased by more than 30%, from 20 billion won in 2020 to 30 billion won in 2021 and further to 40 billion won in 2022.
Last year, its sales exceeded 50 billion won and are now approaching 60 billion won.
Entresto underwent pricing reductions five times until last year, a 21% ↓ from 2243 won to 1774 won Entresto has undergone five price reductions, including adjustments made through the price-volume agreement and voluntary price reduction.
The price of Entresto was initially listed at 2243 won for 500 mg, 1,000 mg, and 2,000 mg in October 2017.
It was subsequently reduced by 1.9% reduction to 2,200 won in September 2019, by 7.0% reduction to 2,046 won in June the following year, and by an additional 6.6% reduction to 1,910 won in February 2022.
In January 2023, the price was reduced by 6.2% to 1,792 won due to the price-volume agreement.
In July of the same year, it was further reduced by 1.0% to 1,774 won due to price adjustments related to expansion of reimbursement.
During this period, the total reduction in drug pricing was 20.9%.
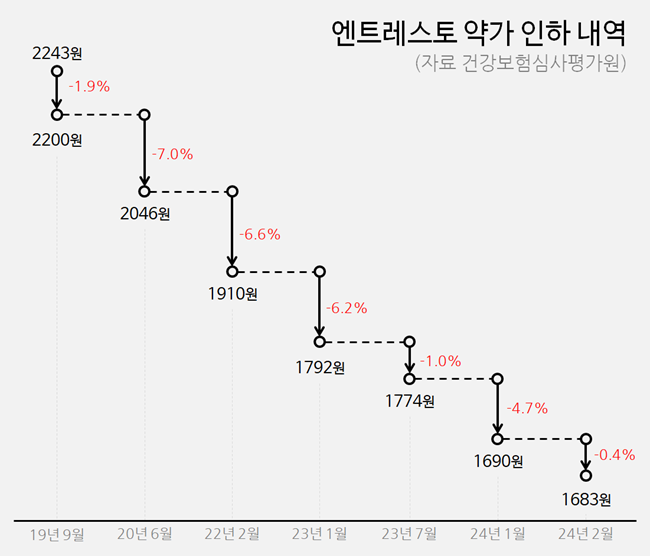
In January last year, it saw a 5% reduction from 1,774 won to 1,690 won due to the volume-price agreement.
In January, Novartis voluntarily reduced the price of Entresto by 0.4% to 1,683 won as Luxturna was reimbursement listed.
The reduction in drug pricing was nullified by additional expansion applications The analysis suggests that despite consistent decreases in drug prices, there has been a significant increase in prescription volume due to expansions in reimbursements.
In 2017, Entresto got reimbursed as a ‘treatment for patients with heart failure with reduced ejection fraction (HFrEF) and decreased heart rate.’ The reimbursement was limited to patients who underwent a combination therapy of standard treatment and stable doses of ACE inhibitor or ARB inhibitor for over four weeks.
In March 2022, Entresto was approved as a first-line treatment, making it available for patients who had not previously been treated with ACE inhibitor or ARB inhibitor.
In July of the following year, Entresto was approved for prescription to both hospitalized patients and outpatients.
There have also been suggestions for the possibility of additional expansion.
In 2021, the United States Food and Drug Administration (FDA) approved Entresto for the indication of treating patients with heart failure with preserved ejection fraction (HFpEF).
Analysis suggests Korea can expect Entresto’s reimbursement to be expanded to include HFpEF, a condition affecting half of the heart failure patients.
Will the upward growth in sales continue?
Factors such as generic companies challenging patents and competing drugs affect this trend The pharmaceutical industry anticipates that Entresto will likely continue its upward trend in sales, especially given the possibility of additional expansion.
There could be two factors affecting this trend.
The first is the outcome of patent challenges by Korean pharmaceutical companies.
A patent dispute related to Entresto is awaiting the Supreme Court’s ruling.
If the Supreme Court rules in favor of the patent-challenging companies, it could lead to the early release of generic versions.
In this case, Entresto’s drug pricing could potentially see a 30% reduction in the first year.
Since 2021, companies like Elyson Pharm have filed successive challenges to Entresto patents, with generic companies winning in all first-instance rulings.
Novartis has appealed three of these cases.
The verdict for one of the appeals was reached in the second instance, ruled in favor of generics as in the first-instance.
Subsequently, Novartis has submitted an appeal to the Supreme Court.
As for Forxiga, AstraZeneca Korea has decided to withdraw Forxiga from the Korean market. Another factor is the emergence of competing drugs.
Last September, Bayer released Verquvo as a reimbursed treatment for chronic heart failure.
This therapy, containing the active ingredient vericiguat, promotes the synthesis of cyclic guanosine monophosphate within cells, regulating heart contraction, vascular tension, and cardiac remodeling.
It represents a novel mechanism for improving myocardial and vascular function.
By the end of last year, prescription sales were approximately 20 million won.
With approval from major hospital pharmacy committees last year, Bayer expects significant prescriptions starting this year.
Additionally, SGLT-2 inhibitor drugs are considered potential competing products for Entresto.
Last year, Forxiga (dapagliflozin) and Jardiance (empagliflozin) received approval for the indication of chronic heart failure.
However, their reimbursements are currently limited to patients with heart failure accompanied by diabetes.
Industry experts anticipate that these two drugs will receive expanded reimbursement for treating patients with chronic heart failure and reduced ejection fraction regardless of having diabetes.
The government is analyzing the financial effects related to expanding reimbursement for SGLT-inhibitor drugs.
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.










