- LOGIN
- MemberShip
- 2025-12-23 06:04:29
- Statin+ezetimibe combo mkt size nears ₩1 tril in KOR
- by Chon, Seung-Hyun | translator Kim, Jung-Ju | 2024-02-15 05:58:39
The statin and ezetimibe combination continues to dominate the dyslipidemia treatment market.
Prescriptions have more than tripled over the past 5 years, with the market size approaching nearly KRW 1 trillion.
The rosuvastatin-ezetimibe combination drove market growth, posting more than KRW 600 billion in sales, while the atorvastatin-ezetimibe combination expanded more than threefold after the arrival of its generics.
Prescriptions for pitavastatin-ezetimibe combinations also surged.
According to the market research institution UBIST, the outpatient prescription market for the statin-ezetimibe combinations totaled at KRW 990 billion ($90.9 million) last year.
This is up 24.1% from the KRW 797.6 billion in 2022.
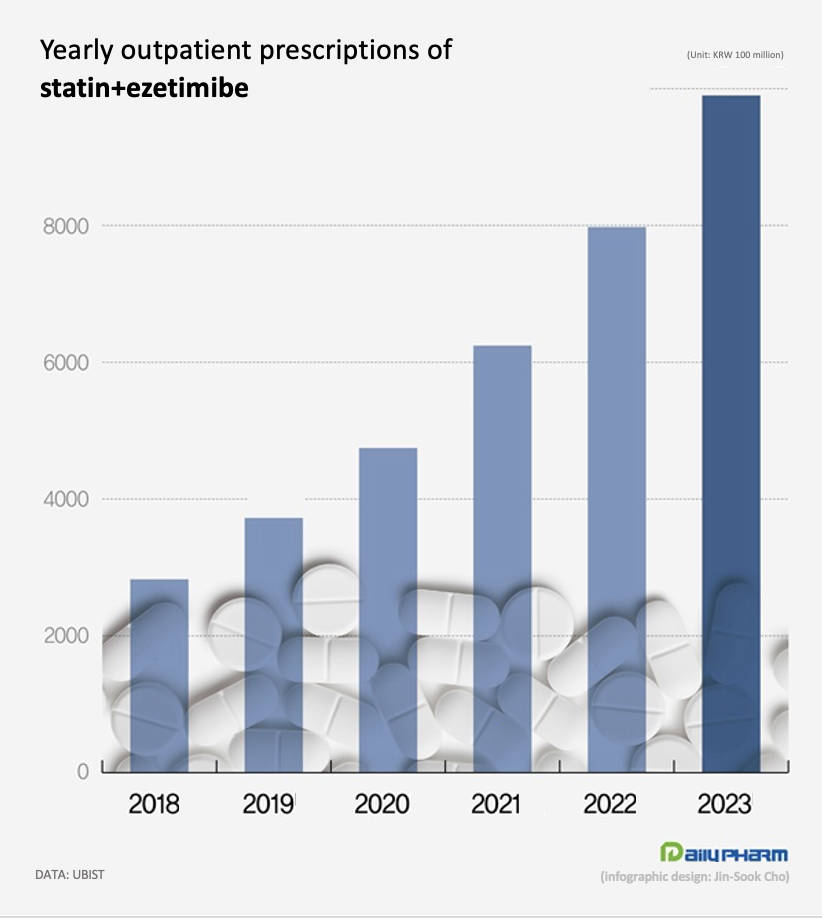
The growth has accelerated recently, more than doubling in the last 3 years from KRW 474.6 billion in 2020.
This is because statin-ezetimibe combinations are more effective in lowering low-density lipoprotein cholesterol (LDL-C) and are less expensive than taking two separate drugs.
Currently, 4 statin-ezetimibe combinations are available: simvastatin, rosuvastatin, atorvastatin, and pitavastatin.
The rosuvastatin-ezetimibe combination owns the largest share of the statin-ezetimibe combination market.
Last year, outpatient prescriptions for rosuvastatin-ezetimibe combinations totaled at KRW 616.4 billion, up 18.7% year-on-year.
This is a 33.1% rise in 2 years from the KRW 450.6 billion in 2021.
Compared to the KRW 197.1 billion it had made in 2018, the market has more than tripled in size in just 5 years.
The rosuvastatin-ezetimibe combination accounted for 62.3% of the statin-ezetimibe combination market last year.
Hanmi Pharmaceutical was the first to enter the market with the rosuvastatin-ezetimibe combination Rosuzet in 2015.
Hanmi entered the market before its competitors by securing the rights to use ezetimibe from the patent holder MSD.
Currently, 50 domestic pharmaceutical companies have entered the rosuvastatin-ezetimibe combination market.
Prescription sales of Rosuzet amounted to KRW 178.8 billion last year, up 19.3% from the previous year, ranking second among all drugs.
This is the third consecutive year it has ranked second since 2021.
Rosuzet has boasted its flagship statin-ezetimibe combination drug status, posting outpatient prescription sales in the KRW 100 billion range for 4 consecutive years, after surpassing the KRW 100 billion mark for the first time in 2020.
Last year, Rosuzet accounted for 29.0% of the rosuvastatin-ezetimibe combination market.
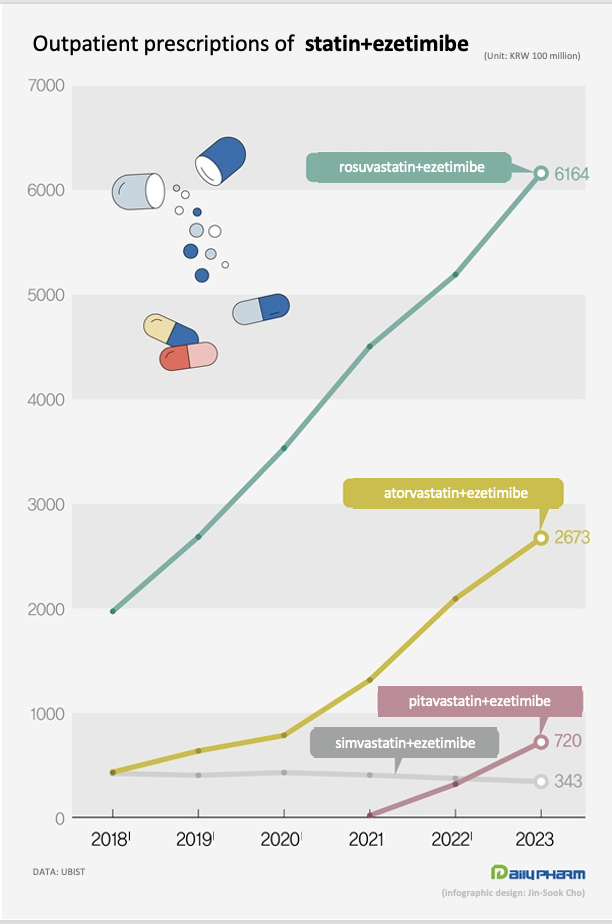
Last year, prescriptions for atorvastatin-ezetimibe combinations totaled at KRW 267.3 billion, up 27.7% year-on-year.
Their sales soared 103.4% in 2 years, from KRW 44.2 billion in 2021 to KRW 131.4 billion.
The market size has expanded rapidly in a short period of time with the introduction of Atozet generics into the market in bulk.
Until 2020, only 1 atorvastatin-ezetimibe combination product – Organon Korea’s Atozet, was available in Korea.
However, Since 2021, more than 100 domestic companies have entered the atorvastatin-ezetimibe market simultaneously, expanding the market size.
In October 2020, Chong Kun Dang received approval for Lipilouzet, a combination drug that contains the same ingredients as Atozet, after clinical trials.
By this time, 22 companies had been approved to produce Lipilouzet-authorized generics and were listed for reimbursement from April 2021.
Beginning in February 2021, 88 pharmaceutical companies additionally received authorizations for Atozet generics and were listed for reimbursement in May, one month later than Lipilouzet-authorized generics.
In June 2021, two more companies received approval for Atozet generics, bringing the total number of domestic companies entering the Atozet market to 113.
The atorvastatin-ezetimibe combination market has expanded by 240.5% in 3 years since the introduction of the generics, up from KRW 82.8 billion in 2020 when only Atozet was available.
The atorvastatin-ezetimibe combination's share of the statin-ezetimibe market rose to 43.3% last year from just 22.0% in 2018.
Livalozet, the first pitavastatin-ezetimibe combination that was launched by JW Pharmaceuticals in 2021, has recently gained prominence in the statin-ezetimibe combination market.
Pitavastatin is the active ingredient in JW Pharmaceutical's flagship hyperlipidemia drug Livalo.
After launched in October 2021, Livalozet posted prescription sales of KRW 31.8 billion in 2022, which more than doubled to KRW 72 billion last year.
Simvastatin-ezetimibe combinations have been less successful.
Last year, prescription of the simvastatin-ezetimibe combinations amounted to KRW 34.3 billion, down 8.0% YoY.
It was the third consecutive year of decline after reaching KRW 42.9 billion in 2020.
Prescriptions for the simvastatin-ezetimibe combinations were down 20.1% last year compared to 3 years ago.
The original simvastatin-ezetimibe combination product, Vytorin from Organon Korea, was the first to enter the statin-ezetimibe combination market.
However, its prescription market has gradually shrunk compared to other combinations.
The simvastatin-ezetimibe combination accounted for 21.4% of the statin-ezetimibe market in 2017, but its share shrank to 5.6% last year.
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.










