- LOGIN
- MemberShip
- 2025-12-23 04:28:21
- Generic Pazeo sales 24%↑ after patent dispute win
- by Kim, Jin-Gu | translator Kim, Jung-Ju | 2024-02-20 06:00:39
Last year, generic products in the market for eye drops with the active ingredient olopatadine, used to treat allergic conjunctivitis, posted a 24% year-over-year (YoY) increase in prescription sales.
It is the most significant sales expansion since 2019.
The pharmaceutical industry anticipates a substantial expansion in prescription sales of generic olopatadine.
In Q3 of last year, generic companies scored a win in a six-year-long patent dispute for ‘Pazeo 0.7% Eye Drops’.
This means that they no longer have the burden of potential infringement of patent and compensation payments.
The prescription of olopatadine eye drops tends to increase in the second and third quarters when outdoor activities are more prevalent.
Therefore, generic companies aim to market a ‘0.7% highly concentrated product’ that no longer has patent risks.
Prescription sales of olopatadine eye drops increased from 57.6 bil to 71.1 bil.
Major generics rose 20%↑ According to UBIST, a pharmaceutical market research agency, on the 19th, the market size of outpatient prescription treatments of allergic conjunctivitis (eye drops) containing the active ingredient olopatadine was 71.1 billion won.
The market has expanded rapidly over five years, with an approximately 14% yearly growth: 39.4 billion won in 2019, 43.6 billion won in 2020, 50 billion won in 2021, and 57.6 billion won in 2022.
Last year, sales increased by 23% YoY, showing a steep growth.
It is speculated that generics, which constitute 86% of the market, performed well, contributing to this growth.
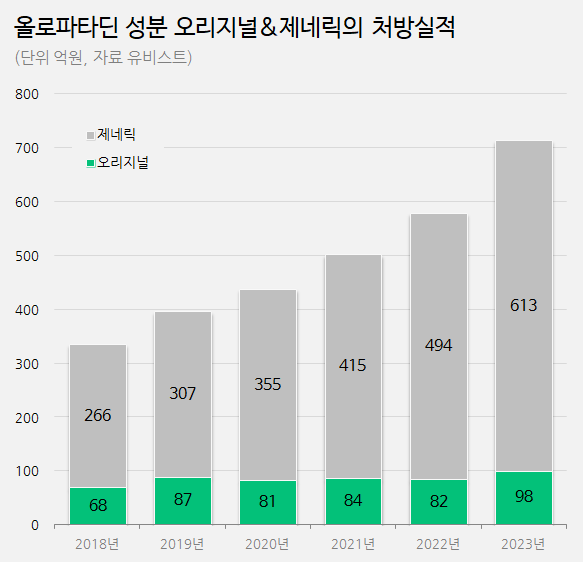
Generics prescription sales of eye drops containing active ingredient olopatadine (Unit: 100 million won, Source: Ubist).
Net prescription sales of generic olopatadine increased from 49.4 billion won in 2022 to 61.3 won last year, showing a 24% growth over a year.
The sales of the major generic products increased by more than 20%.
The sales of Sam Chun Dang’s ‘Oloten'·'Oloten Hi'·'Oloten Plus’ increased from 7.8 billion won in 2022 to 9.4 billion won last year, showing a 20% growth.
The sales of Lite PharmTech’s ‘Lite Olon’ series Eye Drops increased from 4.3 billion won to 6.7 billion won, a 57% growth.
The sales of Hanlim Pharm’s ‘Olo-Once'·'Olopanol'·'Olopower’ increased from 4.1 billion won to 5.9 billion won, a 44% growth.
The sales of Dae Woo Pharmaceutical’s ‘Paradin’ series Eye Drops increased from 4.6 billion to 56 bilion won, a 44% growth.
With the risk associated with patent trials reduced, generic companies will now aggressively market the ‘0.7% high concentration product’ The pharmaceutical industry anticipates that generic prescription sales will likely expand even more this year.
This is because generic companies won against Novartis in a patent dispute for Pazeo Eye Drops, eliminating patent infringement risks.
On August 31st of last year, the Supreme Court dismissed all Alcon’s appeals in the second trial against Hanmi Pharm.
The patent dispute over Pazeo Eye Drops that lasted six years ended with generic companies’ victorious win.
Novartis owns three original products containing the active ingredient olopatadine, differentiated by dosage: 0.1% Eye Drops named ‘Patanol,’ 0.2% Eye Drops named ‘Pataday,’ and 0.7% Eye Drops named ‘Pazeo.’

Novartis filed a separate patent for its 0.7% high concentration product.
The patent dispute was related to Pazeo.
Pazeo, a medication developed by Novartis in 2016, has a higher active ingredient concentration at 0.7%.
Novartis filed two separate active ingredient patents for Pazeo, which will expire in 2032.
Generic companies filed a patent invalidation trial against two patents.
Based on their victory in the second trial, several companies have since launched generics of 0.7% eye drops.
However, after Novartis appealed to the Supreme Court, generic companies did not market their products aggressively.
The analysis suggests that generic companies were cautious about potential patent infringement and subsequent compensation payments if the Supreme Court ruled in favor of Novartis, leading to their passive marketing of the 0.7% product.
There is a noticeable difference in prescription records between the original product and generic products, particularly in terms of concentration.
In the case of the original product, prescription records are highest for the 0.7% high-concentration product.
Based on last year's prescription sales, Patanol, the 0.1% product, and Pataday, the 0.2% product, each amounted to 1.7 billion and 2.4 billion won respectively, while Pazeo, 0.7% product, reached 5.6 billion won.
More than half (57%) of the prescription sales for olopatadine original products comes from Pazeo.
On the other hand, for generics, the prescription sales for the 0.7% product are at a minimal level.
Taking the market-leading product series from Sam Chun Dang, the Oloten series, as an example, while Oloten, the 0.1% product, and Oloten Plus, 0.2% product, each amounted to 3.2 billion and 5.1 billion won, respectively, Oloten Hi, the 0.7% product, only reached 1.1 billion won.
The proportion of the 0.7% product in the entire series is merely 12%.
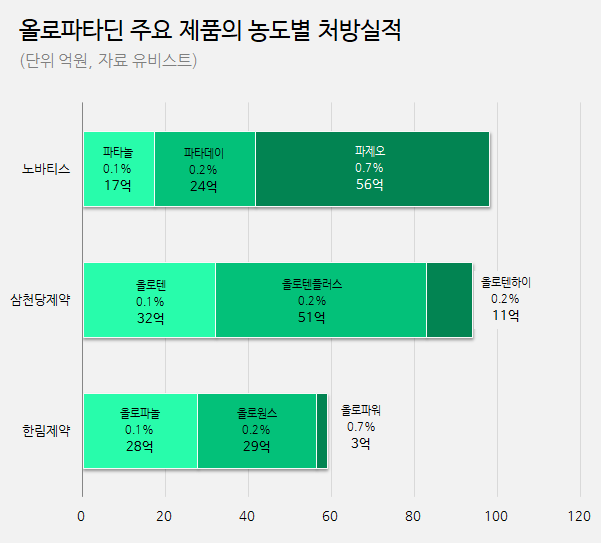
The highest prescription volume for the original product is for 0.7% high concentration product.
For generics, prescriptions are primarily for the 0.1% and 0.2% products. It was observed that Hanlim Pharm, which holds the third-largest share in the market, had a similar record of prescription pattern.
Last year, the prescription sales for their 0.1% product, Olopanol, and 0.2% product, Olo-Once, were 2.8 billion won and 2.9 billion won, respectively, whereas the 0.7% product, Olopower, only amounted to 300 million won, constituting about 4% of the total.
As a result, many companies have opted to launch products with concentrations of 0.1% and 0.2%, instead of the 0.7% product.
However, last year’s Supreme Court ruling has greatly reduced risks involved in trials.
As a result, generic companies are expected to begin marketing 0.7% high-concentration products aggressively.
Sales performance during the second and third quarters will be crucial, as prescriptions are primarily made during the second and third quarters due to the high outdoor acitivities that exacerbate allergic conjunctivitis.
A pharmaceutical company with a related product stated, “We have high expectations for the 0.7% product.
Especially now that the trial risks have diminished, we anticipate aggressive sales this year.”
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.










