- LOGIN
- MemberShip
- 2025-12-23 02:35:53
- Shingrix leads shingles vaccine mkt…occupies 44% of mkt
- by Chon, Seung-Hyun | translator Kim, Jung-Ju | 2024-02-26 05:24:59
The new shingles vaccine, Shingrix, has risen to lead the market in its first year of sales.
It quickly gained 44% of the market share with its strong shingles prevention effect despite its high price.
Since the addition of Shingrix, the market has doubled in size.
According to the drug research institution IQVIA, the shingles vaccine market was worth KRW 87 billion last year, expanding 105.8% from the previous year.
The domestic shingles vaccine market has been declining every year after reaching KRW 89.9 billion in 2019.
In 2022, the market size was KRW 42.3 billion, less than half of what it was 3 years ago.
The analysis is that the pandemic has caused the premium vaccine market to shrink.

In last year’s shingles vaccine market, Shingrix posted the most sales, posting KRW 38.5 billion in sales.
The vaccine, which has been available since December 2022, began to exert its presence in Q1 last year with sales of KRW 6 billion, occupying a 28.9% share.
In Q2 last year, it quickly became the leading shingles vaccine, posting KRW 11.1 billion, and continued to lead in Q3 and Q4, posting KRW 9.9 billion and KRW 11.1 billion, respectively.
Shingrix’s share of the shingles vaccine market last year reached 44.2%.
In other words, the vaccine took the market by storm, occupying nearly half of the total market in its 1st year of release.
In Q4 last year, the company's market share rose to 50.2%, further solidifying its lead.
The greatest benefit of Shignrix is its strong shingles-prevention effect.
In a Phase III clinical trial (ZOE-50) that was conducted on adults aged over 50 years of age, Shingrix showed a 97.2% efficacy compared to the non-vaccinated group at 3.2 years of follow-up, and in another Phase III clinical trial (ZOE-70) conducted on adults aged 70 years and above, Shingrix showed an 89.8% efficacy at 3.7 years of follow up.
This is superior to the 5% protection in adults aged over 50 years of age and 41% in adults aged 70 years and above demonstrated with the use of Zostavax.
The prevention rate of SKYZoster is also known to be comparable to Zostavax.
Also, Shingrix’s safety profile was confirmed through 5 clinical trials that were conducted on immunocompromised patients aged 18 years and older.
Based on such evidence, patients who received autologous hematopoietic stem cell transplantation or those with solid cancer, blood cancer, or solid organ transplant patients who have an increased risk of shingles are also eligible to receive vaccination with Shingrix.
At the time of its release, Shingrix's significantly higher price than existing vaccines was pointed to as an obstacle to the vaccine’s early settlement into the market.
vaccines.
The price of Shingrix, which is administered two times in total, is set at around KRW 500,000 to 600,000.
This is more than twice as high as the existing vaccine, which costs KRW 150,000 to 200,000.
However, Shingrix rapidly increased its market share in the market supported by its superior efficacy despite the high price.
Also, the sales support from 2 domestic pharmaceutical companies contributed to Shingrix’s rapid market penetration.
GSK started domestic sales of Shingrix in partnership with two companies, GC Biopharma and Kwangdong Pharmaceutical.
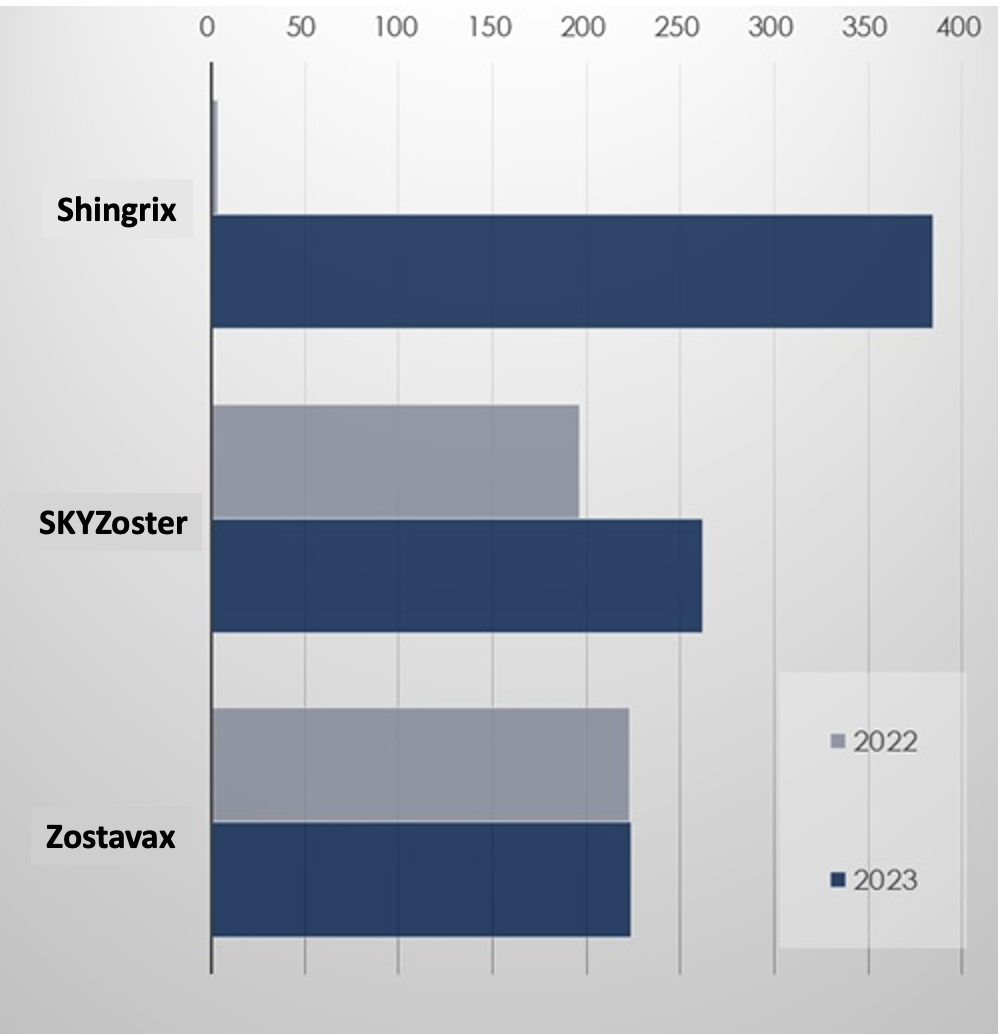
SK Bioscience’s SKYZoster’s sales had risen 33.3% YoY to record KRW 26.2 billion last year.
SKYZoster is a live attenuated vaccine for the prevention of shingles that was developed by SK Bioscience with its proprietary technology.
The vaccine has proven its non-inferiority compared to its competitor (Zostavax) in adults aged 50 or older in 8 domestic clinical institutions.
In October 2017, the company obtained approval for SKYZoster from the Ministry of Food and Drug Safety for 'preventing shingles in adults over the age of 50'.
Before its approval, MSD's Zostavax was the only vaccine in the market, and the introduction of SKYZoster had sparked market competition.
SKYZoster's growth slowed during the pandemic after posting KRW 34.1 billion in sales in 2019.
In 2021, sales fell to 18.2 billion won, a 46.7% drop in two years.
SKYZoster rebounded in 2022 with 19.7 billion won in sales and grew even more last year.
However, its market share fell from 46.5% in 2022 to 30.1% in one year due to the rise of Shingrix.
In the case of Zostavax, it posted sales of KRW 22.4 billion last year, up 0.5% YoY.
In 2019, Zostavax posted KRW 55.9 billion in sales, occupying 62.1% of the market share.
However, its sales have been on a downward trend since then, and its share shrank to 25.7% last year.
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.










