- LOGIN
- MemberShip
- 2025-12-23 04:25:28
- Cash cow ‘bepotastine’ to face reimb re-evaluation
- by Chon, Seung-Hyun | translator Kim, Jung-Ju | 2024-03-06 06:02:54
In facing re-evaluation of ‘bepotastine’ for reimbursement in the upcoming year, pharmaceutical companies fear its impact.
The market size for bepotastine has increased by more than 50% in just two years during the pandemic and endemic, labeling bepotastine as a new cash cow.
However, there are concerns that the companies may face financial loss if drugs containing bepotastine as an active ingredient receive negative results from the re-evaluation, such as reduced reimbursement or removal.
According to industry on the 6th, the Ministry of Health and Welfare (MOHW) recently held the Health Insurance Policy Review Committee, where they reported on drugs that will be re-evaluated for reimbursement appropriateness in 2025.
Next year, the reimbursement appropriateness of eight active ingredients, including olopatadine, clematis radix plus trichosanthes root plus prunella spike, bepotastine, spherical adsorptive carbon, artemisia Herb extract, L-ornithine- L-aspartate, and chenodeoxycholic acid-ursodeoxycholic acid, will be determined through the re-evaluation.
The MOHW explained, “We have selected eight active ingredients.
Five active ingredients have been listed for an extended time, and three active ingredients are currently under clinical re-evaluation by the Ministry of Food and Drug Safety (MFDS)." The MOHW will perform a comprehensive analysis and evaluation of drugs based on clinical research article results, clinical usefulness, cost-effectiveness compared to substitute drugs, and the overall increase in insurance benefits for society.
The compiled data will be reviewed by experts, and the MOHW will decide whether to maintain, reduce, or remove reimbursement.
The industry is particularly interested in hearing results from the MOHW’s re-evaluation of the appropriateness of reimbursement for bepotastine because the market has expanded significantly recently.
Pharmaceutical companies may face financial loss if the drugs containing bepotastine receive negative results from the re-evaluation, such as reduced reimbursement or removal of indication.
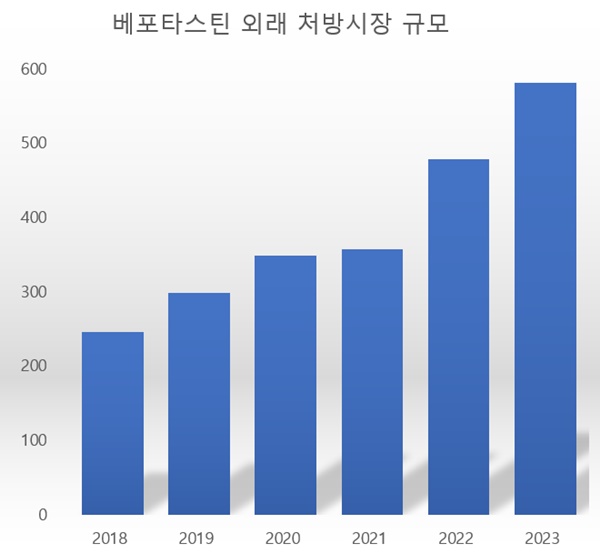
The prescription market for bepotastine experienced rapid growth during COVID-19 and endemic.
According to UBIST, a pharmaceutical market research agency, the out-patient prescription market size for bepotastine was 70.2 billion won last year, an increase of 17.7% from the previous year.
The prescription market size for bepotastine stayed mainly the same between 2018 and 2021, generating 45.3 billion won and 45.3 billion, respectively.
However, it reached 59.7 billion won in 2022, showing a growth of 31.8%, and even greater last year.
The prescription market size for bepotastine expanded to 55.2% in the past two years.
Since the end of 2021, there has been a significant surge in demand for nasal discharge treatment, which is one of the remedies to alleviate symptoms of COVID-19.
This is due to the increasing number of people testing positive for COVID-19, with hundreds of thousands of new cases daily.
Even after the pandemic, there are still many COVID-19-positive patients and growing patients with influenza or the common cold, leading to even greater demand for bepotastine.
Dong-A Pharmaceutical’s Twolion dominates the prescription market for bepotastine.
Twolion’s prescription sales were 10.7 billion won, up 11.7% from the previous year.
In two years, it has increased 33.1% from 8.1 billion won in 2021.
Mitsubishi Tanabe Pharma’s Talion is the original medicine with bepotastine as an active ingredient.
Initially, Dong-A ST marketed Talion, but Mitsubishi Tanabe Pharma withdrew from the Korean market after its patent expired at the end of July.
Since then, Dong-A Pharmaceutical received approval for Twolion, a generic version of Talion, and Dong-A ST is responsible for its marketing.
Daewon Pharm’s Bepostarbi recorded 4.9 billion won in prescription sales last year, a 29.1% increase in just two years since making 3.8 billion in 2021.
Dong Kook Pharmaceutical’s Bepotan accumulated 3.7 billion won in prescription sales, up 47.0% from two years ago.
The prescription sales for Medica Korea’s Galion were merely 1.4 billion in 2021 but recorded 3.3 billion won last year, more than doubling the size.
Only two of the eight active ingredients survived the reimbursement re-evaluation conducted last year by the MOHW.
The MOHW conducted re-evaluation of reimbursement on eight ingredients, including rebamipide, levosulpiride, loxoprofen sodium, limaprost alfadex, epinastine hydrochloride, oxiracetam, acetyl-L-carnitine hydrochloride, and hyaluronic acid (HA) eye drops.
Only two of the ingredients, rebamipide and levosulpiride, maintained their reimbursement prices since the drugs proved clinical usefulness.
Loxoprofen sodium, limaprost alfadex, and epinastine hydrochloride are the three active ingredients receiving reimbursement cuts.
Loxoprofen sodium’s indication, ‘Reducing fever and pain relief for acute upper respiratory inflammation,’ will be excluded from receiving the reimbursement.
For limaprost alfadex, the reimbursement will not cover its indication, one of the two, for ‘Improving ischemic symptoms such as ulcer, pain, and cold sensation that are associated with thromboangitis obliterans.’ Oxiracetam and acetyl-L-carnitine hydrochloride were excluded from the current re-evaluation because the MOHW had already stopped allowing reimbursement for those two ingredients due to inadequate demonstration of their effectiveness based on the MFDS’s clinical re-assessment.
The decision for hyaluronic acid (HA) eye drops has been postponed until reimbursement criteria for all disposable eye drops are established.
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.










