- LOGIN
- MemberShip
- 2025-12-23 02:37:39
- Korean and global pharmas in race for lung cancer drugs
- by Son, Hyung-Min | translator Kim, Jung-Ju | 2024-04-09 05:50:20
Pharmaceutical and biotechnology companies in South Korea are conducting clinical trials to overcome drug resistance in conventional non-small cell lung cancer (NSCLC) therapy.
These companies are developing 4th-generation lung cancer treatments that have proven effective in patients with drug resistance after the use of 1st-to-3rd-generation targeted therapies.
Conventional EGFR-positive NSCLC therapies are categorized into 1st-generation AstraZeneca’s Iressa (gefitinib) and Roche’s Tarceva (erlotinib), 2nd-generation Boehringer Ingelheim’s Giotrif and Pfizer’s Vizimpro (dacomitinib), and 3rd-generation Yuhan Pharmaceutical’s Leclaza (lazertinib) and AstraZeneca’s Tagrisso (osimertinib).
However, drug resistance can still occur when using targeted therapies with proven effectiveness.
The C797S mutation is the most common mutation in EGFR-positive targeted therapies.
Treatment options following the targeted therapies are limited.
Patients with resistance to targeted therapies have the option of using anticancer chemotherapy, docetaxel, or cancer immunotherapy.
However, these drugs do not significantly improve response rates.
Latecomer companies target a C797S mutation that causes resistance after the conventional 1st-to-3rd-generation targeted therapies, aiming to seek commercialization opportunities.
The analysis is that these drugs compete against antibody-drug conjugates (ADC), which have proven effective in patients resistant to targeted therapies, for commercialization.
The K-Bio industry is conducting clinical trials targeting C797S mutation According to industry sources on April 6, domestic biotech venture J INTS BIO presented clinical phase 1/2 results on its 4th-generation EGFR-positive candidate JIN-A02.
Byoung Chul Cho (Director of the Lung Cancer Center at Yonsei Cancer Hospital), who is also in charge of Leclaza and Rybrevant, leads the clinical stage of JIN-A02.
JIN-A02, a 4th-generation EGFR tyrosine kinase inhibitor (TKI), has an underlying mechanism of action that selectively binds to C797S, which causes resistance to 3rd-generation NSCLC treatment.
In the clinical study, JIN-A02 confirmed a partial response (PR) in one patient and stable disease (SD).
J INTS BIO explained that among 4th-generation EGFR-TKI treatments, it is the first instance of PR in patients with the C797S mutation.

A phase 1/2 trial of BBT-207 is currently being conducted, enrolling 90 patients with EFGR-positive NSCLC in South Korea and the United States.
Bridge Biotherapeutics plans to understand data on different mutations in patients acquired through liquid biopsy.
In a preclinical trial, BBT-207 demonstrated anti-tumor effectiveness in various EGFR mutations, including the C797S mutation.
Therapex has received approval from the Ministry of Food and Drug Safety (MFDS) for a phase 1/2 TRX-221 trial last month.
Therapex plans to determine the recommended dose in Phase 1 and assess effectiveness in Phase 2a.
The company aims to obtain approval for the indication in advanced NSCLC with EGFR C797S mutation.
Previously, Therapex demonstrated the drug’s dose-dependent anticancer efficacy and blood-brain barrier (BBB) permeability in a Tagrisso-resistant brain tumor mouse model.
Voronoi has obtained approval for a phase 1 trial in South Korea and is conducting the clinical trial.
Voronoi also targets EGFR C797S.
Through this phase 1 trial, the company plans to evaluate the drug’s effectiveness against the C797S-resistant mechanism of action.
HK inno.N is conducting research on IN-119873, a 4th-generation targeted anticancer treatment, for patients who have shown resistance in the first-line treatment of NSCLC or have an L858R mutation.
Unlike conventional treatments that target the binding site of adenosine triphosphate (ATP), an energy source of cancer cells, IN-119873 targets the allosteric binding site (one of the protein binding sites) of the EGFR, providing a significant advantage.
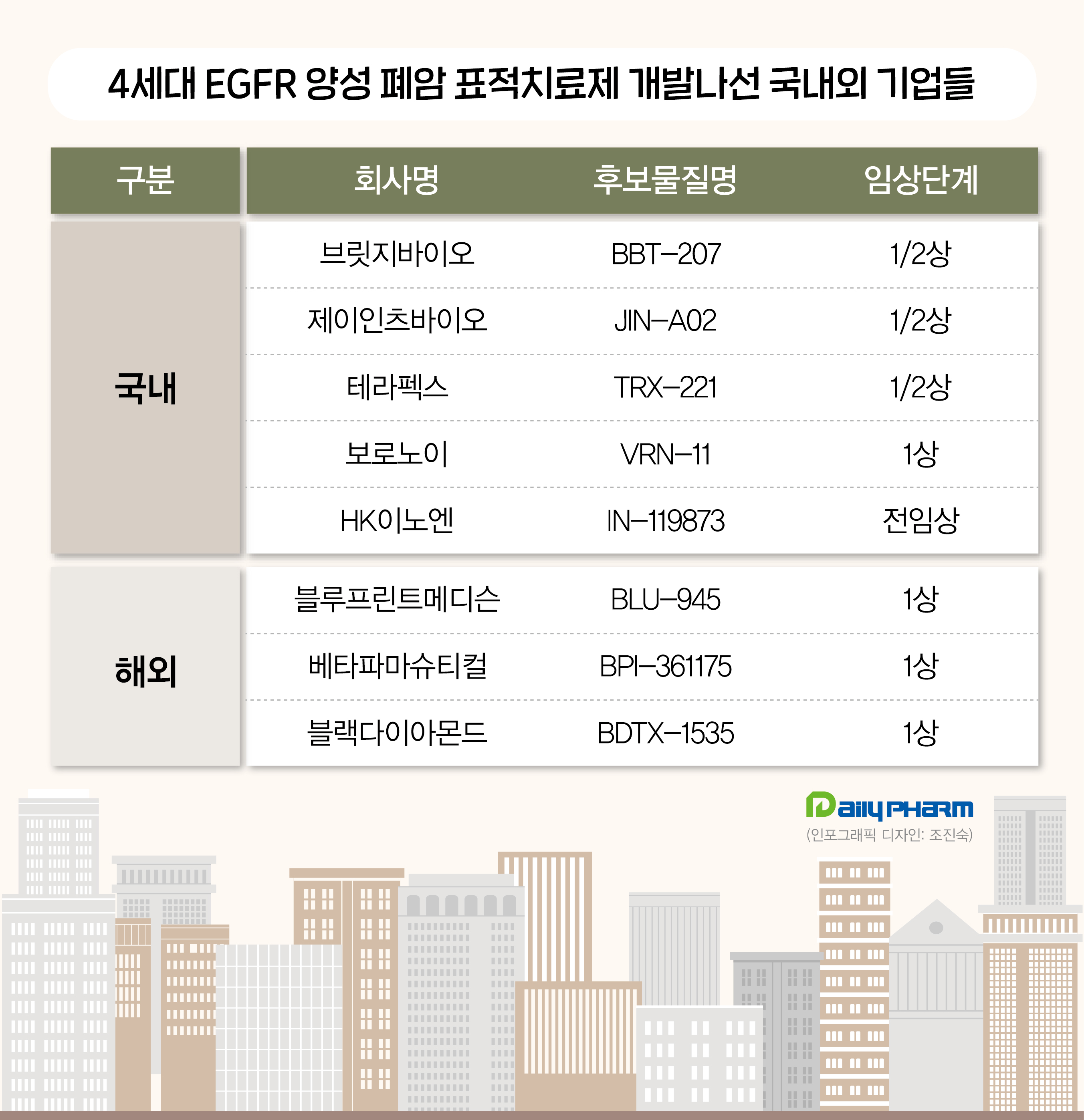
For overseas pharmaceutical companies, Black Diamond Therapeutics leads the clinical race…Will it surpass ADC Overseas pharmaceutical companies, as well as Korean biotechnology companies, are actively conducting clinical trials on 4th-generation lung cancer treatments.
Black Diamond Therapeutics confirmed the highest number of partial responses (PR) in their phase 1/2 clinical trial.
They are repurposing their existing brain tumor treatment, BDTX-1535, as a 4th-generation lung cancer targeted therapy.
In a clinical trial targeting NSCLC patients with acquired resistance to targeted therapy, Black Diamond Therapeutics's 4th-generation EGFR-TKI treatment, BDTX-1535, yielded results of five patients with partial response (PR) and six patients with stable disease (SD) out of a total of twelve.
In contrast, the U.S.-based Blueprint Medicines faces difficulties in drug development as it failed to confirm efficacy in Phase 1 clinical trials.
The company faced setbacks with its 4th-generation targeted therapy candidate, BLU-945 monotherapy.
However, the company is currently exploring the possibility of commercializing it as a combination therapy with Tagrisso.
Blueprint Medicines plans to target exon 21 L858R mutations rather than the C797S mutation in Tagrisso-resistant patients.
However, the commercialization of these targeted therapies faces a challenge in surpassing ADC clinical results.
Currently, Daiichi Sankyo and MSD are jointly developing an ADC that has shown effectiveness in Tagrisso-resistant patients, and their data are being disclosed.
Daiichi Sankyo and MSD’s Patritumab deruxtecan, which targets human epidermal growth factor receptor 3 (HER3), has shown effectiveness in EGFR-TKI patients compared to platinum-based chemotherapy in the phase 2 HERTHENA-Lung01 study.
In the clinical trial, patritumab demonstrated complete responses (CR) and confirmed 66 partial responses (PR).
The objective response rate (ORR) was observed at 29.8%.
Currently, this treatment is designated as a priority review drug by the U.S.
Food and Drug Administration (FDA), with approval expected to be finalized in June of this year.
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.










