- LOGIN
- MemberShip
- 2025-12-23 02:36:39
- SK’s CMO business posted KRW 812 billion last year…
- by Chon, Seung-Hyun | translator Kim, Jung-Ju | 2024-04-11 05:44:46
SK Group's contract manufacturing organization (CMO) business posted a deficit last year.
Sales reached nearly KRW 1 trillion, but its growth was sluggish.
Investments increased due to the expansion of production facilities at acquired companies, and demand for contract manufacture of COVID-19 drugs from overseas pharmaceutical companies decreased.
According to SK on April 9, SK Pharmteco’s sales revenue last year was KRW 812 billion, down 10.5% from the previous year.
SK Pharmteco posted an operating profit of KRW 49 billion in 2022 but turned to an operating loss of KRW 89 billion.
SK Pharmteco is comprised of five entities: SK Biotech, SK Biotech Ireland, AMPAC, Yposkesi, and CBM.
The company has a localization strategy that allows the company to carry out CMO businesses in the U.S.
and Europe by establishing local manufacturing facilities.

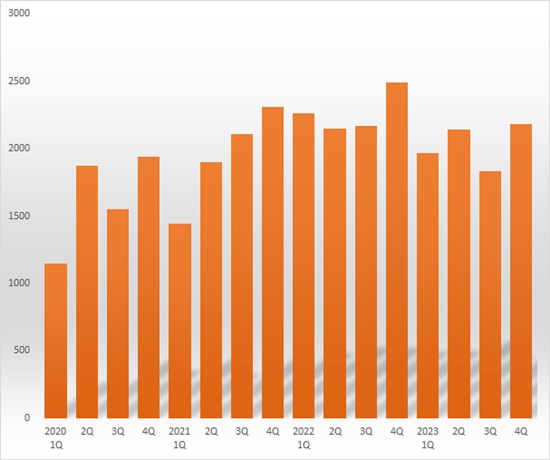
In terms of quarterly sales, SK Pharmteco recorded KRW 249 billion in sales in Q4 2022, followed by KRW 197 billion in Q1 2023, a 20.9% decrease from the previous quarter.
The company posted a loss of KRW 15 billion in Q1 last year.
Sales rebounded to KRW 214 billion in Q2 last year but fell 15.7% YoY to KRW 183 billion in Q3.
The decline in orders for COVID-19 drugs from large pharmaceutical companies created a revenue gap for CDMOs.
SK Pharmteco posted a loss of KRW 15 billion each in Q1 and Q2 last year.
The losses reflected costs related to the expansion of its production facility in Virginia, U.S.
In the Q4 last year, sales reached KRW 218 billion, up 19.1% from the previous quarter.
The company explained, “Sales increased due to the expansion of our pipeline that includes our core products, and the effect of the acquisition of CBM.” The company posted a loss of KRW 59 billion in Q4 last year, which reflects the initial operating loss of the gene cell therapy business following the acquisition of CBM.
SK Pharmteco acquired the management rights for The Center for Breakthrough Medicines (CBM), a U.S.
cell and gene therapy CDMO, in September last year.
In January 2022, SK Pharmteco invested USD 350 million (approximately KRW 420 billion) to fortify its bio business in the U.S.
and became the second-largest shareholder.
It ascended to become CBM's largest shareholder afterward by exercising the call option rights it secured during that time.
CBM is building a 65,000㎡ facility, the world's largest single manufacturing facility for cell and gene therapies, of which approximately 28,000㎡ have been completed to house its Viral Vector GMP facility and development and analytical laboratories.
When the GMP production facility for plasmids, the raw material used for cell and gene therapy drugs, is completed this year, the entire process, including development, production, and analysis, from plasmids to finished products such as viral vectors and cell therapy products, will be provided in one place.
Viral vectors are virus-based gene transfer vectors that insert therapeutic DNA into viruses for safe and efficient administration to the human body.
Compared to using different suppliers for each development and production process, the production period and cost can be reduced.
SK is implementing a strategy at the group level to develop SK Pharmteco into a global CDMO company.
Unlike how Samsung Biologics, whose CMO business has been growing rapidly in recent years, produces and supplies biopharmaceuticals ordered by overseas customers at its Songdo plant in Incheon, SK Pharmteco implemented a localization strategy and built production bases in the U.S., Europe, and other countries to develop its CMO business.
SK Biotech, which is in charge of the domestic production base, was established in April 2015 by spinning off SK Biopharm's raw pharmaceutical material business.
In 2016, SK incorporated SK Biotech as a 100% subsidiary.
SK invested KRW 40 billion in March 2016 and KRW 172.5 billion in November 2017 through a paid-in capital increase.
SK Biotech engages in the business of developing new raw materials using its proprietary technology.
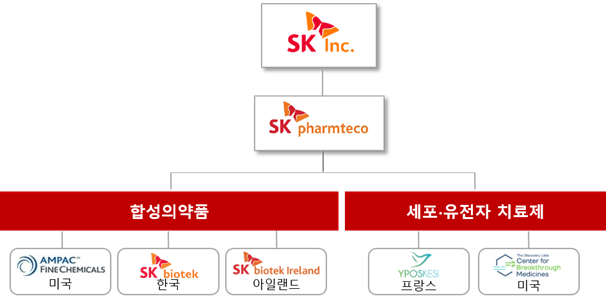
SK Biotech Ireland is the successor of BMS’s Ireland plant, which was acquired by SK Biotech in June 2017 for KRW 170 billion.
In 2019, SK acquired a 100% stake in U.S.
biopharmaceutical CDMO AMPAC to secure a U.S.
manufacturing base.
AMPAC has production facilities in California, Texas, and Virginia.
SK Group invested about KRW 1 trillion to acquire SK Biotech Ireland and AMPAC.
The three entities, SK Biotech, SK Biotech Ireland, and AMPAC, produce synthetic drugs.
In March 2021, SK Pharmteco expanded into the biopharmaceuticals sector with the acquisition of French gene and cell therapy drug contract manufacturer Yposkesi.
Last year, it became the largest shareholder of CMB and secured an additional production base for cell and gene therapy.
SK Pharmteco plans to strengthen its global market penetration through integrated operations of CBM and Yposkesi.
Yposkesi’s second plant was completed in June last year, and totaled at 10,000㎡, the largest in Europe.
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.










