- LOGIN
- MemberShip
- 2025-12-22 23:07:37
- Forxiga prescriptions 'drop' after a 'withdrawal notice'
- by Kim, Jin-Gu | translator Kim, Jung-Ju | 2024-04-25 05:50:27

After announcing its withdrawal from the Korean market at the end of last year, the company has distributed only the remaining stocks of Forxiga in South Korea, which may have impacted prescription sales significantly.
Pharmaceuticals benefiting from Forxiga’s absence include generics, which were launched after Forxiga’s patent expiration last year, and Daewoong Pharmaceutical’s Envlo (ingredient: enavogliflozin), a new diabetes drug that falls into the same class as Forxiga.
As of Q1 this year, Forxiga generics hold a 25% share of the SGLT-2 inhibitor market, while Envlo has expanded to 6%.
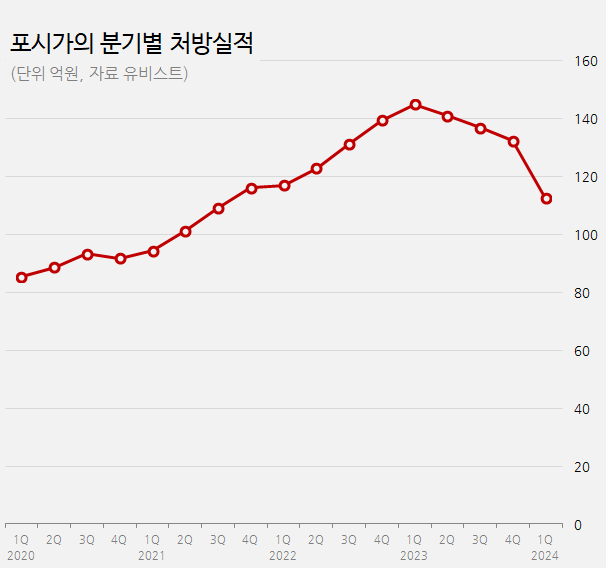
Prescription sales of Foxiga in Q1 dropped by 22%...↑continued decline since the decision to withdraw from the Korean market According to the medical market research firm UBIST, Forxiga’s outpatient prescriptions in Q1 this year amounted to KRW 11.3 billion, a 22% decline over a year compared to KRW 14.5 billion in Q1 last year.
The analysis suggests that the decline in the prescription performance of Forxiga can be attributed to the release of generics following Forxiga’s patent expiration and its withdrawal from the Korean market.
Forxiga has been a top-selling drug in the market since competition in the SGLT-2 inhibitor market competition has started, expanding its prescription performance.
Its sales peaked in Q1 last year, generating prescription sales of KRW 14.5 billion.
Although the government’s 30% price reduction measure in response to generic releases was suspended via administrative litigation, generics containing the same ingredient have been expanding their presence in the market, shaking Forxiga’s position.
Indeed, after the launch of generics, Forxiga’s sales began to trend downward.
The decline in prescription performance in Q1 this year was further influenced by AstraZeneca Korea‘s decision to withdraw Forxiga from the Korean market.
In December last year, AstraZeneca Korea made a decision to withdraw Forxiga from the Korean market.
The company plans to withdraw Forxiga, a monotherapy drug, and only leave 'Xigduo,' a combination therapy drug containing metformin.
Currently, AstraZeneca Korea only provides the existing stock without additional imports from the global headquarters.
In Q1 of this year, supply decreased due to inventory depletion, leading to a significant decline in prescription performance.
Monthly prescriptions for Forxiga decreased over time, from KRW 4.3 billion in December last year to 4 billion in January this year and to KRW 3.6 billion each in February and March.
Forxiga gap, filled by domestic companies in South Korea… with generics accounting for 25% of the market share and Envlo for 6% As Forxiga, the market’s No.1 product, is set to withdraw from South Korea, other products are competing to fill the gap left by Forxiga.
Analysis of the Q1 performance indicates that generics and Daewoong Pharmaceutical’s Envlo are emerging as contenders to fill this gap.
In Q1 this year, Forxiga generics recorded a total of KRW 10.2 billion.
After April last year, 64 Forxiga generics were launched.
These products expanded sales rapidly, generating KRW 3.9 billion in Q2, KRW 6.8 billion in Q3, and KRW 8.3 billion in Q4.
In Q1 this year, Forxiga generics expanded its market share to 25% in the entire SGLT-2 inhibitor monotherapy market (KRW 40.6 billion).
This represents pulling market share to a quarter of the KRW 150 billion market size within just one year of its launch.
Regarding product sales, Boryung’s 'Trudapa' and Hanmi Pharm’s 'Dapalon' have grown significantly.
In Q1, Trudapa recorded prescription sales of KRW 1.2 billion, while Dapalon recorded KRW 1 billion.
Moreover, Aju Pharm’s 'Daparil,' Chong Kun Dang Pharmaceutical’s 'Exiglu,' and Kyung Dong Pharma’s 'Dapazin' have recorded over KRW 500 million in Q1.
However, the other Forxiga generics have very low prescription performance.
In Q1, out of 64 generic products, 59 (92%) had less than KRW 500 million prescription sales.
Among these, 41 products had less than KRW 100 million in quarterly prescriptions.
The average amount prescribed for a generic product is about KRW 159 million.
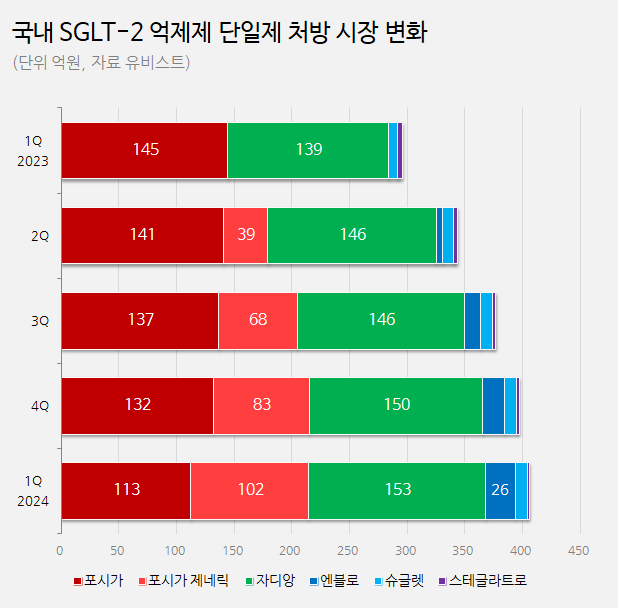
Envlo’s Q1 prescription sales were KRW 2.6 billion (including the prescriptions of HanAll Biopharma’s 'Eaglex').
Envlo is the first SGLT-2 inhibitor developed in Korea, and it was launched in May.
Envlo recorded KRW 500 million in Q2 last year, KRW 1.3 billion in Q3, and KRW 1.9 billion in Q4.
In Q1 this year, it expanded its market share by 6% in the SGLT-2 inhibitor monotherapy market.
Following Forxiga, 'Jardiance,' which ranked second in the market, also benefited.
Jardiance’s Q1 prescription sales were KRW 15.3 billion, up 10% compared to KRW 13.9 billion in Q1 last year, becoming the No.1 in the market.
However, Jardiance’s market share in the SGLT-2 inhibitor market decreased from 47% to 38%, a 9% drop.
While prescription performance increased due to the absence of Forxiga, it is analyzed that it failed to expand market share because of competition from Forxiga generics and Envlo.
The pharmaceutical industry expects the market share of Forxiga generics and Envlo to accelerate further after Q2 this year when Forxiga withdrawal begins in full swing.
AstraZeneca Korea is anticipating the timing of domestic withdrawal once the remaining stock is depleted.
As of Q1, the prescription sales gap, valued at over KRW 10 billion, will be divided between Forxiga generics and Envlo.
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.










