- LOGIN
- MemberShip
- 2025-12-22 23:08:24
- A change in the DPP4 diabetes market worth KRW 600 billion
- by Kim, Jin-Gu | translator Kang, Shin-Kook | 2024-04-26 05:48:22

Following the expiration of substance patents for major original products, generics have been launched, rapidly expanding their prescriptions.
At the same time, the growth of original products is experiencing a slowdown.
The prescription sales of Tenelia (teneligliptin) generics have increased by 83% year-over-year (YoY), resulting in a widening gap between the generics and the originals.
The sales of Galvus (vildagliptin) generics will soon surpass those of the original product.
Moreover, Januvia (sitagliptin) generics have rapidly expanded their market impact since September last year, achieving significant growth in less than six months.
Tenelia generics have grown significantly…prescription sales of KRW 8.3 billion→KRW 15.2 billion 'up' According to the medical market research firm UBIST on the 24th, the market size for prescriptions of DPP-4 inhibitor class diabetes treatment in Q1 was KRW 150.9 billion.
This represents a 4% decrease compared to KRW 156.5 billion in Q1 last year.
Recently, this market has transformed as the patents of major original products have expired one after another.
The patent of Novartis’ Galvus expired first in March 2022, followed by Handok’s Tenelia patent in October of the same year.
In September of last year, the patent of Januvia, which has been a top-selling drug, also expired.
After patents expired, the original products’ generics were released into the market one after another, rapidly expanding their prescriptions.
On the other hand, most original products have faced decreased prescription sales.
Among these, Tenelia generic has shown a significant growth in prescription sales.
Since Tenelia’s patent expiration, 37 pharmaceutical companies have launched generic versions of monotherapy Tenelia and combination therapy Tenelia M.
The Q1 prescription sales of these generics were KRW 15.2 billion, which is an 83% YoY increase.
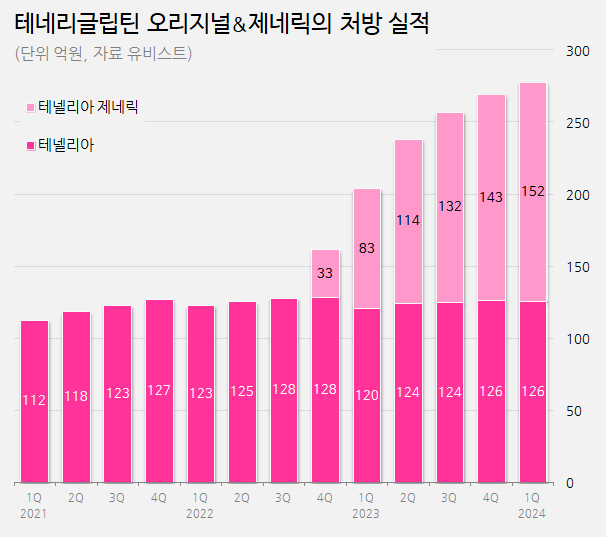
generics (unit: KRW 100 million, source: UBIST). In Q2 last year, Tenelia generics exceeded KRW 10 billion in net prescription sales, and by Q3, they had surpassed the original product sales.
The gap between the sales of generics and original products continues to widen.
In Q1 this year, generics expanded their market share to 55% in the diabetes treatment market containing teneligliptin.
On the other hand, the original products, Tenelia and Tenelia M, show a slowdown in performance.
In Q1 of this year, the combined prescription sales of these two original products amounted to KRW 12.6 billion, a slight increase from KRW 12.4 billion in the same period last year.
The prescription performance of Tenelia and Tenelia M expanded until Q3 of 2022, just before the patent expiration, reaching KRW 12.8 billion, and plateaued after that.
Generics of Galvus·Januvia are gaining more market share…while the sales of original products slow down Galvus generics are also gradually gaining influence and will soon surpass the original product.
In Q1, the combined prescription sales of Galvus·Galvusmet were KRW 6.1 billion, an 8% increase compared to KRW 5.7 billion YoY.
During the same period, prescription sales of the original products decreased by 7% from KRW 7.4 billion to KRW 6.9 billion.
The quarterly prescription sales of Galvus increased to KRW 12 billion just before patent expiration, but it has steadily declined since the release of generics.
As the generic prescriptions increased and that of the original drugs decreased, the gap between them significantly narrowed.
The difference between the originals and generics, which stood at KRW 1.7 billion in Q1 last year, narrowed by KRW 700 million over the year.
In the market for diabetes treatments containing vildagliptin, the generic market share expanded from 44% to 47%.
Galvus generics are expected to outperform the originals by the end of the year.
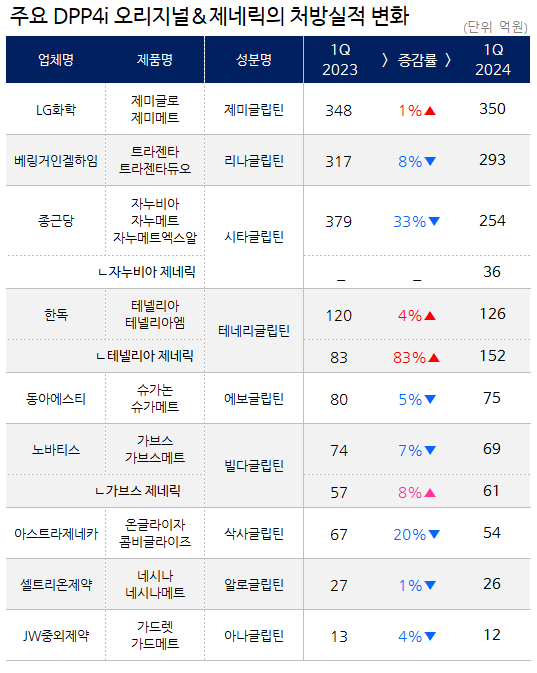
generics. In Q1, the combined prescription performance of Januvia and Janumet generics reached KRW 3.6 billion.
Januvia's patent expired in September last year.
Over 100 companies obtained approval for related generics before the patent expiration.
Since Januvia maintained the leading position in the DPP-4 diabetes market, with prescription sales exceeding KRW 160 billion annually until just before the patent expiration, the product attracted much attention from generic manufacturers.
After the patent expiration, more than 50 companies have launched competing products.
The prescription sales of original products Januvia·Jaumet·Janumet XR decreased by 33% over the past year, dropping from KRW 37.9 billion to KRW 25.4 billion due to the release of generics and consequent price reductions.
In May, before Januvia's patent expired last year, Chong Kun Dang Pharmaceutical acquired all domestic rights to the Januvia series from MSD.
The total contract amount was KRW 45.5 billion.
Chong Kun Dang Pharmaceutical paid KRW 23 billion upfront to MSD headquarters and an additional USD 17 million (approximately KRW 22.5 billion) based on sales milestones.
Zemiglo maintains the leading position in the DPP-4 market…Januvia·Trajenta sales have been steadily decreasing Due to a rapid decline in the Januvia series' prescription sales has intensified competition for market leads.
LG Chem’s Zemiglo (gemigliptin)·Zemimet maintained their leading position by recording prescription sales of KRW 35 billion in Q1 last year.
Although the Zemiglo series was introduced to the market later than multinational pharmaceutical products, it has experienced rapid growth and surpassed KRW 30 billion in quarterly prescription sales in Q3 of 2020.
In Q3 of 2021, Zemiglo surpassed the Trajenta series, which recorded prescription sales of KRW 34.1 billion, by generating prescription sales of KRW 34.5.
Then, in Q3 last year, it even surpassed the Januvia series to take the lead in the market.
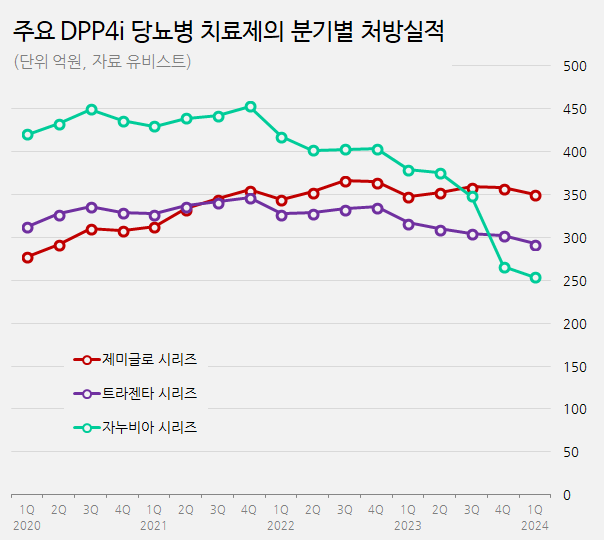
The prescription sales of these two products in Q1 of this year amounted to KRW 29.3 billion.
Compared to Q1 of last year, which recorded KRW 31.7 billion, there has been an 8% decrease over the year.
Their prescription sales are ranked second in the market but have a noticeable long-term downward trend.
The quarterly prescription sales of the Trajenta series have been steadily decreasing since reaching a peak of KRW 34.6 billion in Q4 of 2021.
Trajenta’s patent expires in June this year.
More than 60 pharmaceutical companies have obtained generic drug approvals and are waiting to enter the market.
With the release of generics, Trajenta's drug price will eventually decrease when the generic version containing the same active ingredient is listed for reimbursement.
According to analysts, prescription sales may continue to decline.
However, Trajenta has more than five unregistered patents.
It is uncertain whether domestic pharmaceutical companies will release generics immediately after the compound patent expires in June.
Releasing generics without overcoming unregistered patents could raise concerns about patent infringement for domestic pharmaceutical companies.
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.










