- LOGIN
- MemberShip
- 2025-12-22 23:07:36
- 11 years since global entry…K-biosimilars
- by Chon, Seung-Hyun | translator Kang, Shin-Kook | 2024-05-29 05:45:57
The biosimilar products developed by Korean companies are actively tapping into the global market.
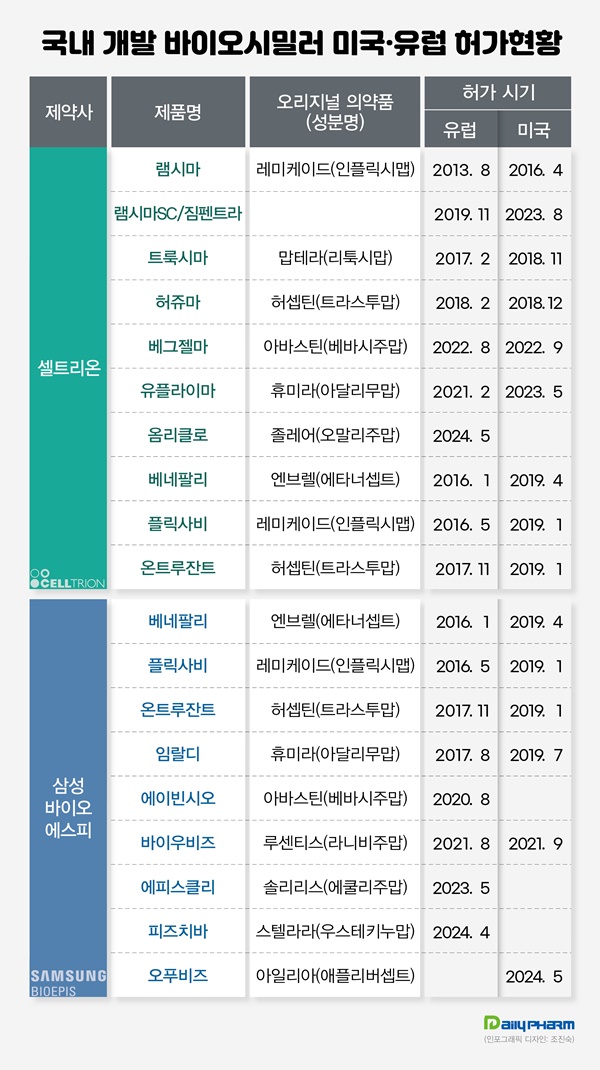
Celltrion received approval for Remsima in Europe in 2013.
Since then, Koran companies have successfully commercialized 15 products in Europe and 12 products in the United States for the past 11 years.
Celltrion secured 11 approvals in Europe and the United States, and Samsung Bioepis accomplished 14 approvals.
According to industry sources on May 27th, Celltrion and Samsung Bioepis’ biosimilars received three approvals from Europe and the United States.
On May 24th, Celltrion received the European Commission (EC) approval for its Omlyclo, a biosimilar to Xolair.
The official approval was granted in two months, after the company received an approval recommendation from the European Medicines Agency’s (EMA) Committee for Medicinal Products for Human Use (CHMP) in March.
Omlyclo is the first Xolair biosimilar to receive European approval.
Xolair is a biosimilar antibody used for persistent allergic asthma, chronic rhinosinusitis with nasal polyps, and chronic spontaneous urticaria.
It recorded KRW 5 trillion in global sales last year.
In Omlyclo’s global Phase 3 trials, which enrolled 619 patients with chronic spontaneous urticaria across six European countries, Celltrion demonstrated Omlyclo’s equivalent efficacy to the original medicine and confirmed a similar safety profile.
In two years since receiving approval for Vegzelma, a biosimilar to Avastin, in 2022, Celltrion established an additional biosimilar pipeline.
This year, Samsung Bioepis received approval for its biosimilar both in Europe and the United States.
Samsung Bioepis received EC approval for Pyzchiva, Stelara’s biosimilar.
The company received the final approval for Pyzchiva two months after receiving a positive review for approval from the EMA’s CHMP in February.
Stelara, developed by Janssen, is prescribed for treating autoimmune diseases, including plaque psoriasis, psoriatic arthritis, Crohn’s disease, and ulcerative colitis.
It inhibits the activities of the immune response-associated inflammatory cytokine called interleukin (IL)-12 and 23.
The annual global sales amount to approximately KRW 1.4 trillion.
Samsung Bioepis added the product to its portfolio after receiving approval for Soliris biosimilar, Epysqli, in Europe in May last year.
Samsung Bioepis received approval from the U.S.
FDA for its Opuviz, a biosimilar to the macular degeneration drug Eylea.
Eylea, developed by Regeneron Pharmaceuticals in the United States, is indicated for the treatment of wet (neovascular) age-related macular degeneration (AMD).
Eylea’s global sales last year amounted to approximately KRW 1.3 trillion.
This marks the approval of Samsung Bioepis’ biosimilar in three years, following the approval of Byooviz, a biosimilar to Lucentis, in 2021.
Celltrion’s Remsima, a biosimilar to Remicade, and others.
Samsung Bioepis’ Benepali, a biosimilar to the autoimmune disease treatment Enbrel, and others. The current list of global entries by companies show that Celltrion has received six approvals in Europe and five approvals in the United States.
Celltrion received the marketing authorization in Europe for its Remsima, titled ‘world’s first biosimilar antibody,‘ in August 2013.
Subsequently, Celltrion received European approval for Truxima, a biosimilar to Mabthera, in 2017 and Herzuma, a biosimilar to Herceptin, in 2018.
These drugs are immunotherapy for cancer.
In November 2019, Celltrion received European approval for Remsima SC, a subcutaneous injection formulation of Remicade, and entered the market.
Remsima SC is a biosimilar developed by Celltrion by changing the formulation of Remicade from intravenous (IV) to subcutaneous (SC) injection.
In February 2021, Celltrion obtained European approval for its Yuflyma, a biosimilar to Humira.
In August 2022, Celltrion obtained European marketing authorization for its Vegzelma, a biosimilar to Vystin.
In 2016, Celltrion's Inflectra, a biosimilar to Remicade, became the first to pass the FDA approval gateway in the United States.
In 2018, Truxima and Herzuma received FDA approval.
In September 2022, Celltrion obtained marketing authorization for Vegzelma, a biosimilar to Avastin, from the FDA, and last year, Yuflyma, a biosimilar to Humira, also received FDA approval.
In August last year, Celltrion received marketing authorization for Zymfentra, a subcutaneous (SC) formulation of the biosimilar antibody Remsima, as a novel drug.
Samsung Bioepis accomplished eight approvals in Europe and six approvals in the United States.
In January 2016, Samsung Bioepis began its global market expansion by obtaining approval for its Benepali, a biosimilar to the autoimmune disease treatment Enbrel, in Europe.
In May of the same year, Samsung Bioepis also obtained marketing authorization in Europe for its biosimilar Flixabi, a biosimilar to the autoimmune disease treatment Remicade.
In 2017, Samsung Bioepis received European approvals for its biosimilars to Herceptin, an immunotherapy for cancer, and Humira, an autoimmune disease medication.
In 2020, the company successfully acquired European approval for its biosimilar to Avastin, and in 2021, it also received a marketing authorization for a biosimilar to Lucentis, an eye disease medication, in Europe.
In May last year, Samsung Bioepis obtained marketing authorization from the EC for ’Episcli,’ a biosimilar to the orphan drug ’Soliris.’ Episcli is the first biosimilar developed by Samsung Bioepis in hematology.
Soliris, developed by Alexion Pharmaceuticals, is a high-cost medication for refractory rare diseases such as paroxysmal nocturnal hemoglobinuria (PNH) and atypical hemolytic uremic syndrome (aHUS).
In April 2017, Samsung Bioepis received the first FDA approval in the United States for its Renflexis, a biosimilar to Remicade.
In 2019, Samsung Bioepis received FDA approval for three biosimilars to Herceptin, Enbrel, and Humira.
In January 2019, Ontruzant, a biosimilar to Herceptin, received marketing authorization in the United States, followed by Eticovo and Hadlima, biosimilars to Enbrel and Humira, in April and July of the same year, respectively.
Samsung Bioepis obtained FDA approval for its Byooviz, a biosimilar to Lucentis, in the United States in September 2021.
Koran companies have successfully commercialized 15 products in Europe and 12 products in the United States for the past 11 years, following Celltrion’s European approval for Remsima in 2013.
The companies strengthened global targeting, entering over two products annually into the United States and Europe.
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.










