- LOGIN
- MemberShip
- 2025-12-22 23:09:49
- More data on Leclaza·Lorviqua showcased
- by Son, Hyung-Min | translator Kang, Shin-Kook | 2024-06-05 05:47:16
Positive clinical results for targeted therapies, such as EGFR and ALK, were featured at the international conference.
Clinical achievements of major targeted therapies for non-small cell lung cancer (NSCLC), including Leclaza, Rybrevant, and Lorviqua, were presented at the American Society of Clinical Oncology (ASCO 2024) annual meeting in Chicago, U.S., on May 31.
For Leclaza, subcutaneous (SC) Rybrevant combined with Leclaza was found to be effective as well as intravenous (IV) Rybrevant combined Leclaza.
Leclaza plus Rybrevant combination therapy has been submitted for U.S.
Food and Drug Administration (FDA) approval as a first-line treatment of EGFR-positive NSCLC.
Pfizer’s Lorviqua, a targeted drug used to treat ALK-positive NSCLC, demonstrated clinical benefit in patients who had no treatment experience.
Based on these clinical results, Lorviqua garners attention for use as a first-line treatment compared to its competitors, such as Alecensa and Alunbrig.
Leclaza combined with SC Rybrevant injection offers benefit The clinical results of Leclaza plus SC Rybrevant combination therapy were presented during ASCO 2024 on May 31st.
This combination therapy is undergoing trials for potential use as a first-line treatment for EGFR-positive NSCLC, and the developer plans to secure ease of administration as well.
Unlike the oral formulation of Leclaza, Rybrevant was developed as an IV injection.
For Rybrevant IV inj, patients have the inconvenience of visiting the hospital once every 2-3 weeks, and the administration takes more than an hour.
Janssen plans to develop an SC formulation to offer ease of administration and reduce concern regarding adverse reactions related to injection.
SC formulation is expected to improve patient convenience since it can significantly reduce the administration duration to within 10 minutes.
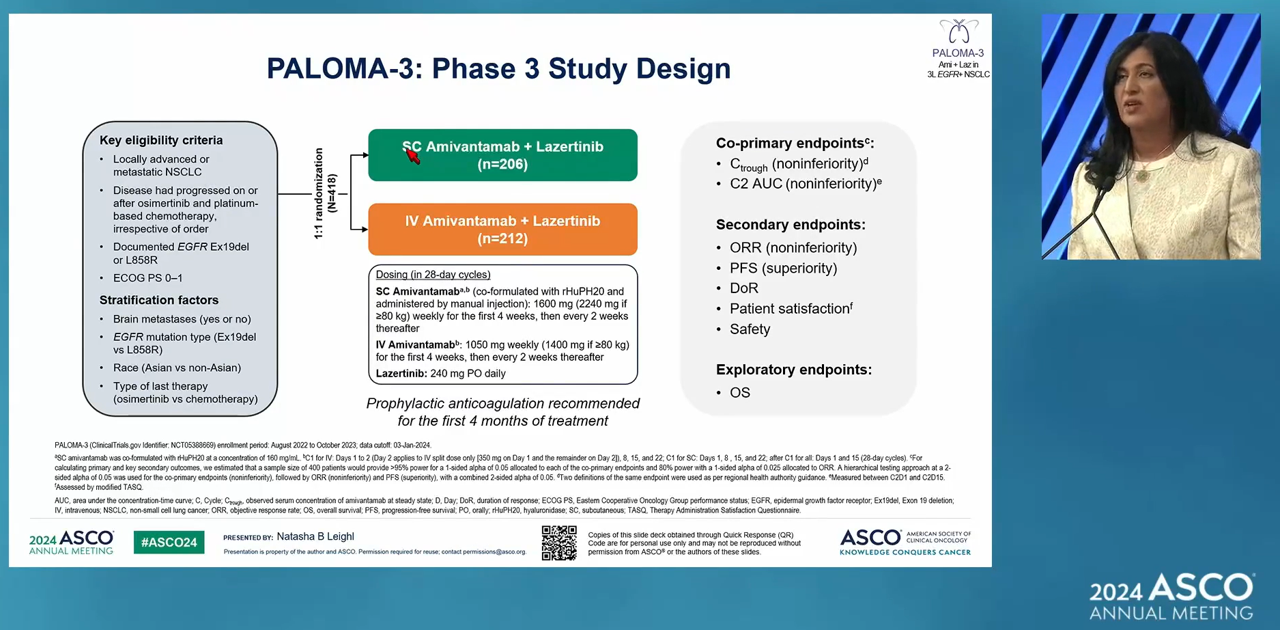
The study enrolled 418 patients with advanced or metastatic NSCLC who have EGFR exon 19 deletions or exon 21 mutations.
The primary endpoint of the study was the non-inferiority assessment of Leclaza and SC Rybrevant combination therapy in pharmacokinetics aspect.
The secondary endpoints included objective response rate (ORR), progression-free survival (PFS), and duration of response (DOR), patient satisfaction, and safety.
At a median follow-up of 7 months, Leclaza plus SC Rybrevant combination therapy showed non-inferiority compared to Leclaza plus IV Rybrevant combination therapy.
Leclaza plus SC Rybrevant combination therapy had an ORR of 30.1%, whereas Leclaza plus IV Rybrevant combination therapy had an ORR of 32.5%, meeting the non-inferiority requirement.
Leclaza plus SC Rybrevant combination therapy showed a positive trend in terms of PFS.
For infusion-related reactions (IRR), Leclaza plus SC Rybrevant combination therapy had 13% of IRR, which was significantly lower than the 66% of IRR for Leclaza plus IV Rybrevant combination therapy.
Whether the SC formulation therapy would overcome the IRR adverse reactions observed in the MARIPOSA study, which evaluated the efficacy of Leclaza plus IV Rybrevant combination therapy is to be watched.
Five-year follow-up data of Lorviqua have been disclosed…60% of patients had PFS

Benjamin J.
Solomon presented the CROWN clinical study results, which confirmed Lorviqua’s five-year survival benefit as the third-generation targeted therapy for ALK-positive cancer (source: snapshot of ASCO 2024 lecture presentation). On May 31st, the five-year follow-up clinical data of Lorviqua, a targeted drug for the treatment of ALK-positive NSCLC, were disclosed at ASCO 2024.
The CROWN Phase 3 study compared the efficacy of Lorviqua to Xalkori for the first-line treatment of ALK-positive NSCLC.
Lorviqua is Pfizer’s third-generation targeted drug for the treatment of ALK-positive NSCLC.
Along with Lorviqua, Takeda’s second-generation Alunbrig and Roche’s Alecensa compete in the market for targeted drugs for treating ALK-positive NSCLC.
As Lorviqua’s effectiveness has been confirmed in five-year long-term data, all eyes in the industry are on whether it will take the lead in the competition among first-line treatments.
The clinical trial involved 296 patients with ALk-positive NSCLC who had no prior treatment experience.
The patients were randomly assigned to the Lorviqua treatment group and the Xalkori treatment group by a 1:1 ratio.
According to the clinical result, during the follow-up period of 60.2 months, the Lorviqua treatment group did not reach the median PFS value.
The Xalkori treatment group recorded 9.1 months of PFS during the follow-up for 55.1 months.
For the five-year PFS rate, 60% of the Lorviqua treatment group reached the PFS, whereas 8% of the Xalkori treatment group reached the PFS.
Furthermore, 77% of the Lorviqua treatment group had Grade 3-4 adverse reactions (AE), whereas 57% of the Xalkori treatment group had Grade 3-4 AE.
The safety profile was aligned with that observed in the previous analysis.
Dr.
Benjamin J.
Solomon, Professor of Peter MacCallum Cancer Centre in Australia, said, “Based on the results of the five-year follow-up data of Lorviqua and Xalkori treatment groups, the Lorviqua treatment group’s media PFS value has not been reached.
This is the longest reported PFS among advanced NSCLC.” He added, “Without any additional safety issues, this will be the unprecedented outcome among ALK-positive NSCLC clinical results.”
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.










