- LOGIN
- MemberShip
- 2025-12-22 23:08:21
- "Breast cancer treatments received positive reviews"
- by Son, Hyung-Min | translator Kang, Shin-Kook | 2024-06-07 05:50:45

ADCs under development by global pharmaceutical companies have shown to be effective in various types of breast cancer, including triple-negative breast cancer, hormone-positive (HR+)/HER2-negative breast cancer.
With these results, latecomers have established a foundation to secure indications for breast cancer, following the cases of Kadcyla, Enhertu, and Trodelvy.
According to industry sources on June 4th, clinical achievements of several ADCs, including Padcev, datopotamab deruxtecan, and sacituzumab tirumotecan, were presented at the American Society of Clinical Oncology (ASCO 2024) annual meeting, which started on May 31st.
Astellas and Seagen have presented clinical outcomes of their ADC Padcev.
Padcev, an ADC anticancer agent targeting the cell surface protein nectin-4, has been approved worldwide for urothelial carcinoma.
During this meeting, the results of the phase 2 EV-202 clinical study, confirming its potential in breast cancer, were disclosed.
Both companies are exploring possibilities not only in urothelial carcinoma but also in breast cancer, gastric cancer, and non-small cell lung cancer, as nectin-4 protein is expressed in various solid tumors.

The primary endpoint was objective response rate (ORR), and the secondary endpoints were duration of response (DOR), disease control rate (DCR), progression-free survival (PFS), and and safety/drug tolerance.
The clinical results showed that the Padcev treatment group had an ORR of 19.0% for triple-negative breast cancer, while the DRR, an index measuring the percentage of patients with no disease progression during or after the treatment, was 57.1%.
Such results were consistent with the outcomes for HR+/HER2- breast cancer.
The Padcev treatment group recorded an ORR of 15.6%, while the DCR was 51.1%.
The treatment-related adverse events (TRAE) of over Grade 3 for Padcev were decreased neutrophil counts (7%), decreased white blood cell counts (5%), and increased aspartate aminotransferase (5%).
Adverse reactions identified in two cohorts were manageable and consistent with previously disclosed safety data.
Currently, for triple-negative breast cancer, there is no ADC approved after Trodevly.
All eyes are on whether Padcev would secure an indication for treating triple-negative breast cancer through follow-up clinical results.
A TROP2-targeting ADC demonstrated additional effects on breast cancer On June 2, the clinical results of datopotamab deruxtecan, under development by Daiichi Sankyo and AstraZeneca, and MSD’s sacituzumab tirumotecan were disclosed.
These two drugs are ADCs targeting TROP2 protein, an intracellular calcium signal transducer regulating cell proliferation and survival.
TROP2 protein is expressed in healthy cells but commonly overexpressed in cancer cells.
The protein is also associated with drug resistance.
Gilead Sciences’ Trodelvy is the only available drug with a similar mechanism that succeeded in commercialization.
Datopotamab is under clinical trials to confirm its potential for breast cancer and non-small cell lung cancer.
The TROPION-BREAST01 study showed that datopotamab improved PFS for HR+/HER2- breast cancer.
This time, the report detailing the study’s patient reported outcome (PRO) was disclosed.
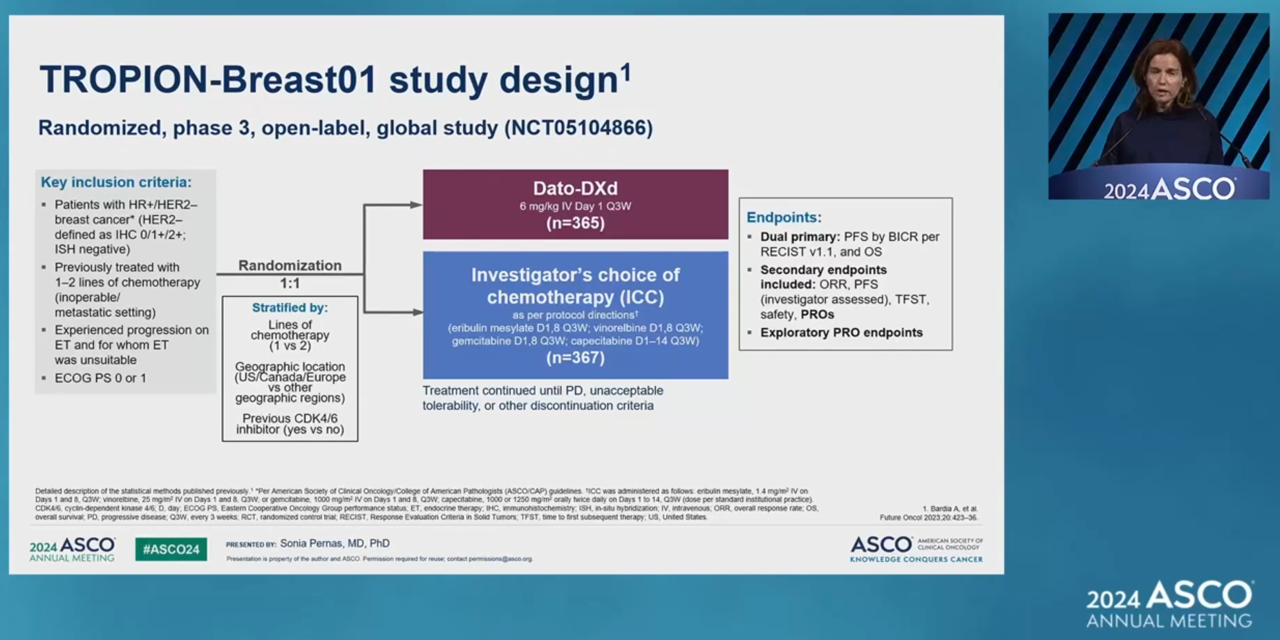
The patients were randomly assigned to either the datopotamab group or investigator’s choice of chemotherapy (eribulin, vinorelbine, capecitabine, or gemcitabine) with a 1:1 allocation.
The PRO included (GGS), quality of life (QoL), and time to deterioration (TTD) evaluated by EORTC QLQ-C30, a questionnaire to assess the quality of life of cancer patients.
The clinical results showed that the TTD for QOL in the datopotamab group was 3.4 months, compared to 2.1 months in the chemotherapy group.
TTD for the datopotamab group was confirmed to be delayed in terms of physical function, pain, and most other symptoms and functioning scales.
MSD’s sacituzumab tirumotecan was shown to be effective in triple-negative breast cancer.
In 2022, MSD licensed the ADC candidate sacituzumab from China-based Sichuan Kelun-Biotech.
Results from the phase 3 OptiTROP-Breast01 study compared sacituzumab to investigator’s choice chemotherapy (eribulin, vinorelbine, capecitabine, or gemcitabine) in patients with locally recurrent or metastatic breast cancer.
The primary endpoint was PFS, assessed by blinded independent central review (BICR).
Interim analysis results for PFS showed that the sacituzumab group recorded a PFS of 5.7 months, which was longer than the 2.3 months in the chemotherapy group.
PFS at 6 months was 43.4% for the sacituzumab group and 11.1% for the chemotherapy group.
Sacituzumab reduced the disease progression and mortality risk by 69%.
OS was significantly favorable for Sacituzumab treatment.
“Sacituzumab monotherapy demonstrated clinically meaningful PFS and OS benefits compared to chemotherapies,” an investigator stated.
“Moreover, it demonstrated a manageable safety profile for treating triple-negative breast cancer, which has limited treatment options.”
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.










