- LOGIN
- MemberShip
- 2025-12-22 19:45:46
- JW Pharmaceutical’s new drugs enter clinical trials
- by Son, Hyung-Min | translator Kang, Shin-Kook | 2024-06-21 05:46:55

Recently, the company's STAT3-targeted anticancer drug candidate entered Phase I clinical trials.
In addition to targeted anticancer drugs, the company also owns first-in-class drug candidates in the fields of atopic dermatitis, hair loss, and blood cancer.
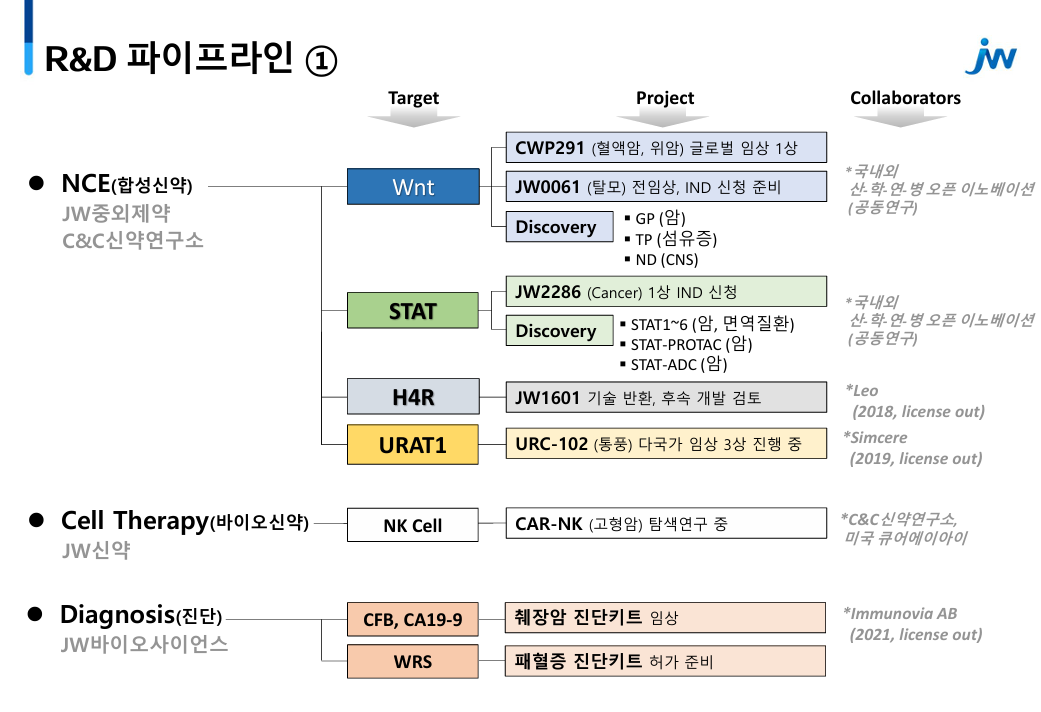
JW Pharmaceutical's competitive edge in drug development is its R&D platform.
JW Pharmaceutical is developing innovative new drugs by utilizing its own platforms JWELRY (JW Excellent LibraRY) and CLOVER (C&C researchLaboratoriesOmics serVER) and 3D cancer organoids secured through open innovation, and artificial intelligence (AI).
3 3 candidates enter clinical trials through the CLOVER platform...secures many first-in-class new drug candidates According to industry sources on the 21st, JW Pharmaceuticals received approval for its IND (investigational new drug application) to initiate a Phase I trial for its targeted anticancer drug candidate JW2286.
The Phase I clinical trial will evaluate the safety and tolerability of JW2286 in 70 healthy Korean and Caucasian adults at Seoul National University Hospital.
In the preclinical trial, JW2286 demonstrated efficacy and safety compared to standard of care in STAT3-overexpressing solid tumors.
In particular, JW2286 showed an effect in hard-to-target triple-negative breast cancer.
JW2286 is a first-in-class drug candidate that selectively inhibits STAT3.
STAT3 is known to be one of the causes of various inflammatory diseases, autoimmune diseases, and cancer, and is highly expressed in various solid tumors, including gastric, colorectal, and triple-negative breast cancers.
In the past, Japanese companies such as Sumitomo Dainippon Pharma and Otsuka Pharmaceutical have been developing STA3-targeted antitumor drugs, but have failed Phase I clinical trials due to lack of efficacy and toxicity issues.
JW Pharmaceutical has secured a clear mechanism of action for targeting STAT3 through its drug discovery platform ‘CLOVER’.
CLOVER can derive small molecules that inhibit or activate the STAT signaling pathway and is regarded as a platform that can perform holistic research on mechanisms and biomarkers.
Through the platform, JW Pharmaceutical has been developing various new drugs through the efficacy of combining candidates with immuno-oncology drugs that utilize the characteristics of tumor immune microenvironment regulation and its biomarker development strategies.
Currently, the company's new drug candidates that have entered clinical trials include JW2286, JW1601 for atopic dermatitis, and URC102 for gout.
Histamine is a neurotransmitter involved in allergic reactions.
However, JW1601 reportedly failed to meet its primary endpoint in a global Phase II trial.
As there are no atopic dermatitis drugs with this mechanism, it would have been the first new drug in its class if developed but was unable to reach the commercialization stage.
JW Pharmaceutical plans to review the future development direction for JW1601 with the possibility of securing new indications in mind.
URC102 is a best-in-class gout drug being developed by JW Pharmaceutical.
The new drug candidate has a mechanism of action that inhibits urate transporter 1 (URAT-1), which allows uric acid to be absorbed back into the body and discharged well.
It was approved for a Phase III clinical trial in Korea in 2022 and has entered clinical trials in China and Taiwan to confirm its commercialization potential.
In addition to STAT3 anticancer drugs, JW Pharmaceutical is also exploring the possibility of developing a STAT3-targeted atopic dermatitis drug, STAT5/3 dual-targeted anticancer drug, and an antibody-drug conjugate (ADC) using the CLOVER platform.
Makes bid into the development of first-in-class new drugs in the field of hair loss and blood cancer JW Pharmaceutical has secured the first-in-class new drug candidates 'CWP291' and 'JW0061' in the fields of hair loss and blood cancer.
The two drug candidates, which target the Wnt signaling pathway, were derived from JW Pharmaceutical's ‘JWELRY’ platform.
JW Pharmaceuticals has been studying the Wnt signaling pathway since the early 2000s and has accumulated data to develop the JWELRY platform.
Wnt plays an essential role in cell proliferation or differentiation, and organ development and morphogenesis in animals.
Wnt pathway inhibition inhibits the formation, proliferation, and metastasis of cancer cells in various tissues, inhibits cancer stem cell activity, and has an anti-fibrotic effect.
On the contrary, activating the Wnt pathway is known to be involved in tissue regeneration by inducing stem cell and cell proliferation.
JW Pharmaceutical is developing JW0061, a new drug candidate for hair loss, through the activation of the Wnt signaling pathway.
The preclinical trial results that have been recently released show that JW0061 has demonstrated superiority in hair follicle production and hair growth compared to existing standard treatments.

Results showed that both low and high doses of JW0061 accelerated hair growth compared to standard of care.
JW Pharmaceuticals is also developing CWP291, which targets blood cancers by inhibiting the Wnt signaling pathway.
CWP291 is a targeted anticancer drug that inhibits Wnt/β-catenin signaling.
The potential of the drug candidate is being studied for multiple cancers, including acute myeloid leukemia, multiple myeloma, and gastric cancer.
According to the Phase Ia trial results to date, one complete response (CR) and one partial response (PR) were confirmed among 54 patients with acute myeloid leukemia with CWP291.
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.










