- LOGIN
- MemberShip
- 2025-12-22 19:42:34
- 'Increased' competitiveness in autoimmune diseases
- by Son, Hyung-Min | translator Kang, Shin-Kook | 2024-06-27 05:47:47
The Korean biopharmaceutical industry is successfully signing license agreements of drugs for autoimmune diseases.
HK inno.
N, IMBiologics, and Y-Biologics have signed license agreements for their jointly developed novel drug candidates.
AprilBio has also signed a license agreement for a novel drug candidate that has shown efficacy in a phase 1 trial.
This is not the first time Korean companies have successfully entered into license agreements for novel drugs for autoimmune diseases.
LG Chem, Voronoi, Hanall Biopharma, and Daewoong have also completed technology transfers overseas.
Even after out-licensing, these treatments have demonstrated efficacy, confirming South Korea’s R&D capacity.
Autoimmune diseases occur when the body’s immune system attacks healthy cells after mistakenly recognizing them as antigens rather than exterior antigens, such as germs and viruses.
Since the cause of these diseases is still unknown, targeted treatments are not available.
As a result, there is an unmet need for more new treatment options.
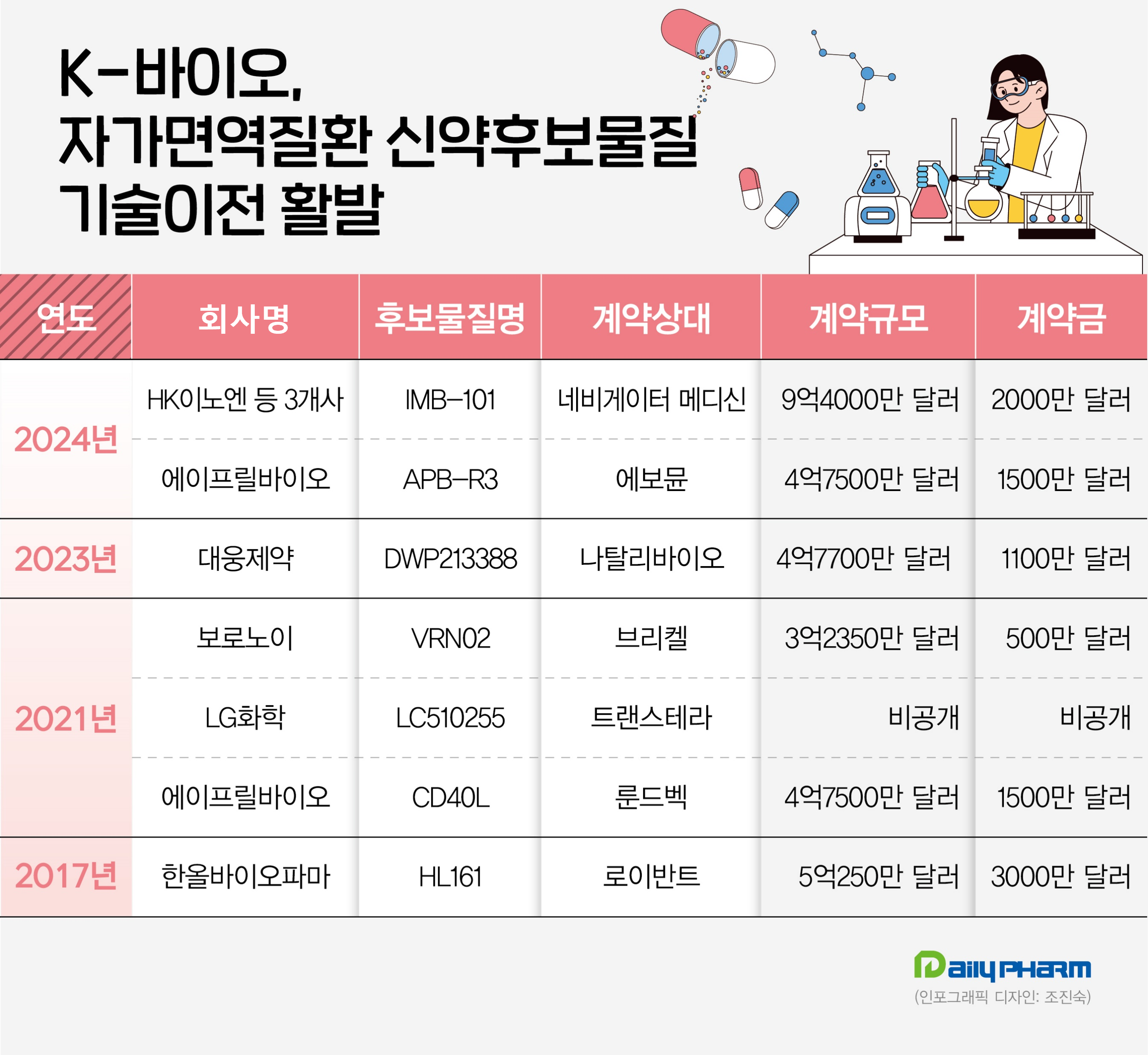
N signed a license agreement with Navigator Medicines, a U.S.
new drug development company, for 'IMB-101,' a novel drug candidate for the treatment of autoimmune diseases.
The contract totaled US$940 million (approximately KRW 1.3 trillion), including an up-front payment of US$20 million (KRW 27.6 billion).
Navigator Medicines secured global development and sales rights through this agreement, excluding Asia.
IMB-101 was jointly developed by HK inno.
N and Y-Biologics in 2016, and it was transferred to IM Biologics in 2020.
The current agreement was led by IM Biologics, a bioventure company established by people who worked at HK inno.N.
IMB-101 is a novel bispecific antibody candidate that is designed to target both OX40L and TNF, simultaneously modulating adaptive and innate immune responses.
OX40L pathway is involved in activating T cells, and TNF is a cell signaling protein involved in immune responses.
Until now, no novel drugs have targeted both OX40L and TNF.
IBM-101 and Sanofi’s SAR442970, in a phase 2 trial, are the only two novel drug candidates that have entered clinical trials.
IM Biologics received approval from the U.S.
Food and Drug Administration (FDA) to conduct Phase 1 trials for 'IMB-101' in August of last year.
The company is currently conducting a Phase 1 trial.
Phase 1 trial will evaluate the safety and drug tolerance of the drug in healthy adults and in patients with autoimmune diseases.
AprilBio has also successfully signed a license agreement with Evommune, a novel drug development company in the United States, for 'APB-R3,' a novel drug candidate for the treatment of autoimmune diseases.
The deal was valued at a total amount of up to US$475 million (approximately KRW 655 billion), including a non-refundable up-front payment of US$15 million (KRW 20.7 billion).
The sales royalty will be paid separately.
APB-R3 is a biologics candidate targeting interleukin (IL)-18.
So far, there are no commercialized products with similar mechanisms of action.
Once APB-R3 is commercialized, it will become the first-in-class.
AprilBio has confirmed the drug tolerance and safety of APB-R3 in a phase 1 trial.
Evommune plans to conduct a phase 2 trial of APB-R3 in the first half of next year.
This marks the second time AprilBio has signed a license agreement for novel drug candidates for treating autoimmune diseases.
In 2021, it out-licensed APB-A1 to Danish pharmaceutical company Lundbeck for US$448 million (approximately KRW 540 billion).
APB-A1 is a novel drug candidate that inhibits CD40L..
CD40L is commonly expressed in activated T-cells due to inflammations.
When T Cells’ CD40L binds to CD40 of natural killer cells, large quantities of cytokines are released.
APB-A1 works by targeting CD40, inhibiting the formation of cytokine-releasing antibodies through B cells and natural killer cells.
Now, UCB’s dapirolizumab and the U.S.
biotech company Horizon Therapeutics’ Dazodalibep are under development with mechanisms of action similar to APB-A1.
Increased out-licensing of novel drugs for autoimmune diseases…clinical trials are making good progress Clinical trials conducted by companies that have developed novel drug candidates for autoimmune diseases are going well.
Hanall Biopharma is developing a subcutaneous formulation of a drug candidate for FcRn antibody therapy batoclimab (HL161).
In 2017, Hanall Biopharma out-licensed HL161, a drug candidate for the treatment of autoimmune diseases, to Roivant Sciences, a Swiss company that also owns Immunovant.
The company is investigating the potential of batoclimab for treating various autoimmune diseases, including myasthenia gravis (MG), thyroid eye disease (TED), immune thrombocytopenic purpura (ITP), neuromyelitis optica (NMO), and chronic inflammatory demyelinating polyradiculoneuropathy (CIDP).
The efficacy of batoclimab has been confirmed in a phase 2 trial, and it is currently undergoing multinational phase 3 trials.
Hanall Biopharma plans to present its top-line results within this year.
LG Chem is developing an autoimmune disease pipeline, ‘LC510255.’ In 2021, the company successfully out-licensed the pipeline to China’s TransThera Biosciences.
The drug is undergoing a phase 2 trial involving patients with ulcerative colitis and atopic dermatitis.
The drug tolerance and satey of LC510255 have been confirmed in a phase 1 trial.
Daewoong Pharmaceutical is developing a novel drug called 'DWP213388' for the treatment of autoimmune diseases.
DWP213388 has a bispecific mechanism of inhibiting both BTK and ITK, which are involved in activating immune cells such as B cells and T Cells.
The phase 1 trial is being conducted in the United States.
Daewoong Pharmaceutical has proven its potential by signing a global license agreement with Vitalli Bio of the United States for DWP213388 at the Korea-U.S.
Digital and Bio-Health Business Forum, which was held in Boston, U.S., last year.
The agreement amounted to US$477 million, including an up-front payment of US$11 million, excluding a royalty payment.
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.










