- LOGIN
- MemberShip
- 2025-12-22 15:43:38
- Yuhan acquires new drugs from biotech venture firms
- by Chon, Seung-Hyun | translator | 2024-08-09 05:34:29
Yuhan significantly increased its research and development (R&D) investment.
Over the past five years since acquiring new drug technology from biotech venture companies, Yuhan's R&D investment size has reached its peak.
This expansion of outward investment seems to be the company's outlook for securing a new portfolio.
According to Yuhan on July 31st, the company's R&D cost in Q2 amounted to KRW 53.5 billion, up 39.8% from KRW 38.2 billion year over year (YoY).
It increased 17.0% from the previous quarterly investment amount of KRW 45.7 billion.
Yuhan's quarterly R&D investment amount exceeded KRW 50 billion in four years since Q4 2020.
In 2020, its R&D investment spending significantly increased due to conducting the global Phase 3 trial for the new anticancer drug Leclaza monotherapy.
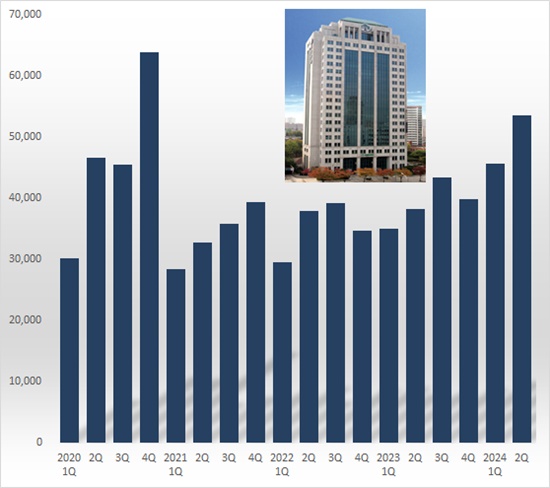
In Q2, Yuhan invested KRW 8 billion in two biotech ventures.
In March, Yuhan signed contracts with Cyrus Therapeutics and KANAPH Therapeutics to acquire technology transfer of anticancer drug candidates based on the SOS1 inhibitor mechanism.
The contract value amounted to KRW 208 billion, including milestones for future development, approval, and sales.
Cyrus Therapeutics is a biotech venture developing targeted anticancer drugs and targeted protein degraders through medicinal and pharmaceutical chemistry-based technology.
KANAPH Therapeutics is developing the next-generation new drug for the field of cancer and autoimmune diseases based on pharmaceutical convergence technology.
The SOS1 inhibitors that Yuhan acquired are anticipated to increase treatment effectiveness in synergy with KRAS inhibitors or EGFR inhibitors and help solve tolerance to conventional therapies.
SOS1 is a protein regulating the activity of RAS, involved in cell proliferation, and it is regarded as a promising target for anticancer function regardless of various RAS mutant types or cancer types.
KRAS and EGFR mutations are common causes of lung cancer, colorectal cancer, pancreas cancer, and cancers with unmet medical needs.
Targeting these is expected to have significant potential in terms of the market.
Cyrus Therapeutics and KANAPH Therapeutics discovered non-clinical candidate products through joint research.
At the American Association for Cancer Research (AACR) conference last year, they presented results showing advantages in pharmacological properties, including superior anti-cancer efficacy in xenograft animal models compared to competitive drugs and improved drug dynamics within the body.
In the second quarter, Yuhan Corporation paid KRW 3 billion in royalties to the biotech company J INTS BIO.
J INTS BIO is focused on developing anticancer drugs.
In 2021, J INTS BIO secured a new pipeline by entering into a transfer agreement for a novel drug candidate developed by Dr.
Kwang Ho Lee from the Korea Research Institute of Chemical Technology (KRICT) and Professor Byoung Chul Cho, Director of the Lung Cancer Center at Yonsei Cancer Hospital.
Yuhan entered into a partnership with J INTS BIO by investing KRW 20 billion each in equity in 2021 and 2022.
Last May, Yuhan signed a licensing agreement with J INTS BIO for the targeted therapy 'JIN-A04.' Yuhan has secured exclusive global rights to develop and commercialize J INTS BIO's tyrosine kinase inhibitors that target HER2 and EGFR.
The technology transfer agreement has a total contract value of KRW 429.8 billion, with a non-refundable upfront payment of KRW 2.5 billion.
On August 5th, Yuhan received approval from the U.S.
Food and Drug Administration (FDA) for the Phase 1/2 Investigation New Drug (IND) under the code name YH42946.
The study will evaluate the safety, drug tolerance, pharmacokinetics, and anti-tumor activation following oral administration of YH42946 in patients with locally advanced or metastatic solid cancers harboring HER2 mutation and EGFR exon 20 insertion mutations.
Yuhan focuses its R&D investment on acquiring external technology to uncover new growth opportunities.
A prime example of Yuhan's success in open innovation is its cancer drug, Leclaza.
Yuhan obtained the development rights for Leclaza from Oscotec and its subsidiary, Genosco, in 2016, just before the drug reached the preclinical stage.
The total contract value was KRW 1.5 billion.
Under the terms of the agreement, the partner received a fixed technology fee of KRW 1 billion within 30 days of the contract signing and an additional KRW 500 million upon approval of Phase 1 clinical trials.
Yuhan entered into a global licensing agreement with Ubix Therapeutics for prostate cancer treatment in May.
Ubix Therapeutics is a Korean biotechnology company developing new anticancer drugs.
Yuhan secured global exclusive rights from Ubix Therapeutics to develop and commercialize a targeted protein degradation (TPD) agent that degrades androgen receptor.
The contract value was up to KRW 150 billion.
An upfront payment was KRW 5 billion, and Ubix Therapeutics could earn up to KRW 145 billion based on development, approval, and sales milestones.
The contract also requires Yuhan to pay royalties based on the net sales of the drug it directly markets.
Additionally, if Yuhan enters into a third-party technology transfer agreement for the product, the revenue from such a technology transfer will be distributed according to the drug's development stage when the agreement is signed.
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.










