- LOGIN
- MemberShip
- 2025-12-22 15:43:06
- Anti-cancer drug trends changes from IV→SC
- by Moon, sung-ho | translator Kang, Shin-Kook | 2024-08-30 05:50:20
Global pharmaceutical companies speed up the development of subcutaneous (SC) formulations of their proprietary intravenous (IV) medications.
To overcome the disadvantage of IV formulation medications that have long administration duration, more anti-cancer drugs are shifting to SC formulations.
Following the trend, Korean companies with SC formulation technology are gaining attention.
Then, what can we predict about the success in real-world clinical practices?
While the shift to SC formulation of anti-cancer drugs is trending, pharmaceutical‧biotech industries focus on real-world clinical practices.
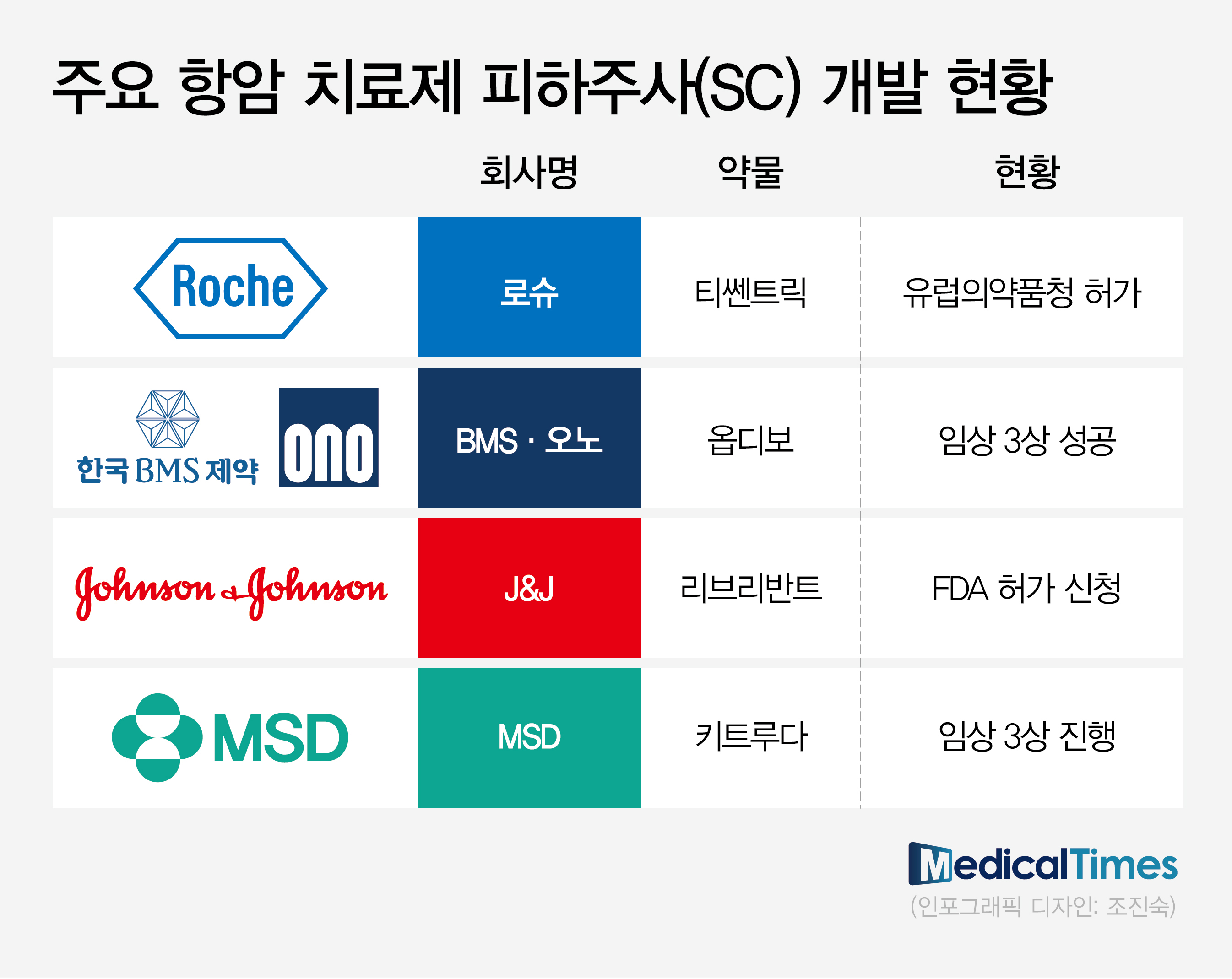
(Table) RocheAccording to pharmaceutical and biotech companies on August 24th, global pharmaceutical companies with proprietary IV formulations of anti-cancer drugs are proactively conducting clinical trials to transition to SC formulation products.
SC formulation products are injected into the subcutaneous layer of the skin.
Injection sites are typically arms, thighs, and abdominal region.
Until now, anti-cancer drugs were available primarily as IV formulations, which inject medications into veins.
IV formulation has the advantages of fast absorption and accurate administration but has the disadvantage of taking a long time.
For IV injection of anti-cancer drugs, patients were burdened by having to visit hospitals and withstand four to five hours of needle insertion.
In contrast, developing anti-cancer drugs as SC formulations has the advantage of substantially improving patient convenience of administration.
The administration duration was reduced from a couple of hours to a maximum of ten minutes.
Therefore, patients do not need to stay in the hospital for a long time for anti-cancer drugs.
As a result, global pharmaceutical companies with immune checkpoint inhibitors are proactively conducting clinical trials and applying for approvals to transition to SC formulations.
Following Roche's 'Tecentriq (atezolizumab)' SC obtaining marketing authorization from the European Medicines Agency (EMA) in January, BMS and Ono Pharmaceutical's R&D of 'Opdivo (nivolumab)' and 'Rybrevant (amivantamab)' is nearing the end.
These immune checkpoint inhibitors share the same goal to defend against their sales decrease due to patent expiration.
Furthermore, the development of SC formulation products has gained more attention since SC formulations have a greater advantage in patient access in the global market, especially in the U.S.
market.
Recently, J&J confirmed the non-inferiority of SC formulations compared to IV formulations through the Phase 3 PALOMA-3 study, presented at the American Society of Clinical Oncology (ASCO) meeting.
Based on the results, J&J has recently applied for additional U.S.
FDA approval on Rybrevant SC formulation.
Professor Byoung Chul Cho (Director of the Lung Cancer Center at Yonsei Cancer Hospital) said, "The United States provides incentives to using injections, and the amount of incentives is the same between IV injectable or SC injectable," and explained, "There is no need to maintain IV formulation injectables, which commonly induce injection-associated adverse reactions." Professor Sun Min Lim (Division of Medical Oncology, Department of Internal Medicine, Yonsei Cancer Hospital) also said, "SC injectables only take 1-2 minutes for Rybrevant administration.
The common adverse reactions of IV injectables are fever and lowered blood pressure," and added, "In my opinion, SC injectables can reduce such adverse reactions." In the global market trend, clinical practices in South Korea are accelerating the introduction of SC formulation products, which overcome the previous disadvantages of IV formulation.
For example, Roche's Phesgo has been recently introduced with reimbursement.
Phesgo is an anti-cancer drug developed by changing the IV formulations of Herceptin (trastuzumab) and Perjeta (pertuzumab) to SC formulations.
It received approval in September 2021 as the first investigational biopharmaceutical.
Phesgo was developed by combining two IV formulation products into SC formulation.
It is known to decrease the administration duration for patients with breast cancer substantially.
It has been designated as an innovative new drug (IND) and is reimbursable, leading to its recent use in clinical practices.
If patients with metastatic HER2-positive breast cancer who have been receiving the maintenance therapy, IV formulations of Herceptin plus Perjeta every three weeks, were to change to Phesgo SC, administration and monitoring duration is expected to decrease from over four hours to 20 minutes.
The remaining step is whether the SC formulation can be used in clinical practices in South Korea.
While there are clear advantages in patient administration, there are opinions that, unlike in the global market, such as the U.S., it is challenging to quickly replace the existing market in South Korea due to geographic accessibility.
Additionally, many believe it will be challenging for healthcare professionals to readily switch to the SC formulation, which they have yet to experience.
However, some argue that the fact that most cancer drug administrations are carried out primarily in large hospitals and are performed in the same way in injection rooms is more positive.
The large number of patients who can receive treatment may facilitate the rapid establishment of the SC formulation.
Professor Park Yeon Hee (Division of Hematolology-Oncology at Samsung Medical Center) said, "Korean patients tend to wait in the hospital, and there are long waits in large hospitals.
Therefore, patients may prefer switching to SC formulations," and stated, "In clinical practices, patients may prefer IV injection despite the wait as SC prescriptions, other than clinical studies, have only been made recently."
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.










