- LOGIN
- MemberShip
- 2025-12-22 15:44:40
- Challenges in the evolving lung cancer treatment landscape
- by Son, Hyung-Min | translator Kang, Shin-Kook | 2024-08-28 05:51:53
The head-to-head battle between the epidermal growth factor receptor (EGFR)-mutated non-small-cell lung cancer drugs (NSCLC) Leclaza and Tagrisso has carried on to competition of each drug's combination therapy regimens.
This month, the U.S.
Food and Drug Administration (FDA) approved Leclaza+Rybrevant as a first-line treatment for EGFR-positive NSCLC.
This is the first time a targeted therapy plus targeted therapy combination has been approved.
Tagrisso was approved in Korea and the U.S.
this year after confirming its efficacy in combination with platinum-based chemotherapy.
While attention is focused on whether combination therapies will find a place in the first-line treatment market for EGFR-mutated NSCLC, there is a consensus that the choice of a first-line treatment should be based on the patient's comprehensive needs, including side effects, frequency of hospital visits, and quality of life.
Leclaza+Rybrevant combo improved overall survival compared with Tagrisso monotherapy
Leclaza is a third-generation tyrosine kinase inhibitor (TKI) that targets exon 19, and exon 21 (L858R) in EGFR-positive NSCLC.
Rybrevant is a targeted treatment option that targets the exon 20, MET mutation.
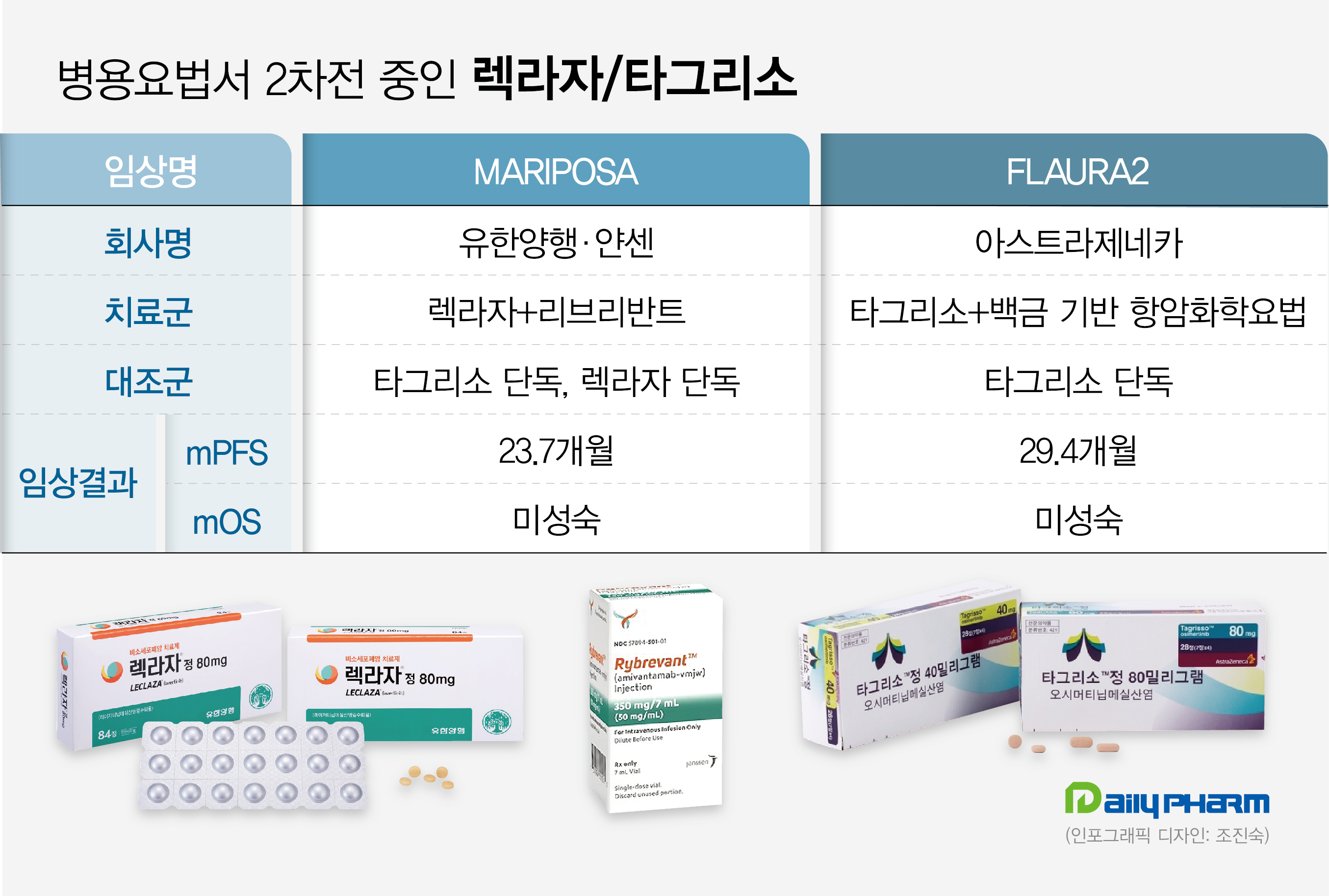
The two companies successfully secured FDA approval for Leclaza+Rybrevant with the MARIPOSA Phase III trial.
Specifically, the FDA approval was based on clinical results presented by Janssen at the European Society for Medical Oncology Annual Congress (ESMO 2023) last year.
The results showed a median PFS of 23.7 months in the Leclaza+Rybrevantcombination arm, which was longer than the 18.5 months in the Leclaza monotherapy arm, and 16.6 months in the Tagrisso monotherapy arm.
Leclaza+Rybrevant combination was associated with a 30% lower risk of disease progression and death than Tagrisso monotherapy.
The interim OS analysis showed a trend favorable to Leclaza+Rybrevantover Tagrisso monotherapy.
PFS2 results showed a 25% lower risk of disease progression or death in the Leclaza+Rybrevant combination arm compared with Tagrisso monotherapy arm.
Tagrisso+platinum-based chemotherapy is approved in Korea and the U.S.
In May, the Ministry of Food and Drug Safety approved Tagrisso+platinum-based chemotherapy as a first-line treatment for EGFR-positive non-small-cell lung cancer.
Tagrisso is a third-generation TKI developed by AstraZeneca.
The Phase III FLAURA2 trial enrolled 557 patients with locally advanced or metastatic NSCLC who had received no prior systemic therapy and were positive for EGFR exon 19 deletion or exon 21 mutation.
The study evaluated the efficacy and safety of Tagrisso combination therapy versus Tagrisso monotherapy.
Results showed that Tagrisso+platinum-based chemotherapy reduced the risk of disease progression or death by 38% compared to Tagrisso monotherapy.
Median investigator-assessed PFS was 25.5 months, an 8.8-month extension compared to 16.7 months with Tagrisso monotherapy.
Median PFS by blinded independent central review (BICR) was 29.4 months, compared with 19.9 months in the Tagrisso monotherapy arm.
Also, inpatients with the L858R mutation, Tagrisso+platinum-based chemotherapy showed a median PFS of 24.7 months, 10.8 months longer than the 13.9 months achieved in the Tagrisso monotherapy arm.
However, side effects management will be a key issue due to its combined use with new drugs.
Rybrevant is an intravenous (IV) formulation that requires dosing once every three weeks.
Adding an IV formulation to the existing oral formulation, Leclaza, could double the effectiveness but reduce dosing convenience.
Janssen is also developing a subcutaneous (SC) formulation to significantly reduce dosing time and address concerns about infusion-related side effects.
Recently published clinical results showed that the combination of the subcutaneous formulation of Rybrevant SC+Leclaza achieved similar outcomes to Rybrevant IV+Leclaza.
At a median follow-up period of 7 months, the Leclaza+Rybrevant SC was non-inferior to Leclaza+Rybrevant IV.
“The Leclaza+Rybrevant combination therapy has continued to generate positive data in brain metastases, L858R mutation, etc., and therefore can be a viable first-line treatment option for EGFR-mutated NSCLC,” said a professor of medical oncology at a university hospital.
”It is difficult to say that use of the combination therapy is good for all patients, as the use of combination therapy is associated with more side effects than using either drug as monotherapy.
This is why prescribing Rybrevantto to older patients can be challenging.” “The use of the intravenous formulation of Rybrevantrequires much attention.
While a subcutaneous formulation is currently in development, the existing intravenous formulation is associated with frequent rashes, paronychia, and infusion-related adverse events.
When selecting a treatment, we should consider not just the efficacy but the patient's overall experience, including side effects, frequency of visits, and quality of life.
So there will be an active debate about which patients will benefit most from the early use of the combination therapy.” Concerns also rise on the lack of later-line therapies following the use of Tagrisso+platinum-based chemotherapy The use of Tagrisso and platinum-based chemotherapy as first-line treatment may lead to a shortage of treatment options for patients who develop resistance.
In the past, EGFR-mutated NSCLC targeted therapies have been used as follows: 3rd generation TKIs were used on patients who were confirmed to be T790M-positive during biopsy after using 1st and 2nd generation TKIs, and platinum-based chemotherapy (Alimta plus carboplatin/cisplatin) was used on T790M-negative patients.
So if a 3rd-generation TKI monotherapy is used in the first line, platinum-based chemotherapy is often used as a second-line treatment as they cannot use 1st or 2nd-generation TKIs due to resistance.
This suggests that the use of both Tagrisso and platinum-based chemotherapy in the first line may lead to a lack of later-line treatment options.
After developing a resistance to the combination, only taxane drugs such as docetaxel and paclitaxel and immuno-oncology drugs targeting PD-L1 will remain as treatment options.
“After failing treatment with EGFR-TKIs, we usually use platinum-based chemotherapy, Alimta+cisplatin/carboplatin,” says a professor of medical oncology at a university hospital.
“Combining these drugs with 3rd-generation TKIs is certainly therapeutic.
However, we should also consider the lack of later-line treatment options after the patient develops TKI resistance if we pull the next line of treatment in advance amid a shortage of treatment options.” “As 3rd-generation TKIs have settled as the first-line standard of care, the role of 1st- and 2nd-generation TKIs will continue to diminish, and it is likely that it will ultimately become a competition between 3rd-generation TKI monotherapy and combination therapy.”
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.










