- LOGIN
- MemberShip
- 2025-12-22 15:48:30
- Pharma companies jump into developing 'TPD' for cancer
- by Son, Hyung-Min | translator Kang, Shin-Kook | 2024-09-03 05:53:23
The global and Korean pharmaceutical industries are focusing on securing Targeted Protein Degradation (TPD) technology, which has emerged following the success of Antibody-Drug Conjugates (ADCs).
Recently, major global pharmaceutical companies such as Pfizer, Novartis, and Eli Lilly have successfully acquired TPD technologies, marking TPD as a new anti-cancer drug development strategy, following targeted therapies, immune checkpoint inhibitors, and ADCs.
Korean pharmaceutical and biotech industries are also exploring the potential of TPD drug development and have achieved successful technology transfers.
According to industry sources on September 3rd, several Korean biopharmaceutical companies, including Yuhan Pharmaceutical, SK Biopharmaceuticals, Daewoong Pharmaceutical, Samjin Pharmaceutical, and Orum Therapeutics, have jumped into developing novel TPD drugs.
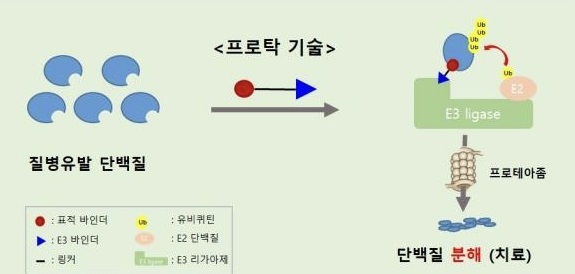
TPD drugs are the next-generation new drug candidate that can use an intracellular protein degradation system to degrade protein of interest specifically.
Unlike conventional small-molecule targeted drugs that inhibit protein function, novel TPD drugs offer superior therapeutic effects and avoid tolerance by directly degrading and eliminating disease-causing proteins.
TPD drugs can target over 80% of disease-causing proteins that conventional small-molecule drugs could not modulate.
Last year, the company invested KRW 62 billion to acquire Proteovant, securing TPD technology.
Founded in March 2020 in the U.S., Proteovant is a biotech venture with global-level technology in the TPD field and molecular glue technology.
Yuhan Pharmaceutical is also eyeing the opportunity to develop TPD drugs as a follow-up to its non-small cell lung cancer treatment (NSCLC), Leclaza.
The company acquired the prostate cancer drug candidate 'UBX-103' in July from Ubix Therapeutics.
As a result, Yuhan Pharmaceutical has secured the lead for clinical trials and exclusive rights for the development and commercialization of UBX-103.
The recently acquired 'UBX-103' by Yuhan Pharmaceutical is a novel prostate cancer drug candidate based on TPD technology.
UBX-103 works by degrading the androgen receptor, overexpressed or hyperactivated in prostate cancer patients.
In the non-clinical studies, UBX-103 showed ten times superior androgen receptor degradation and suppression of prostate cancer cell proliferation in wild type and mutant compared to 'ARV-110' of Arvinas, a U.S.-based company.
In a castration-resistant prostate cancer mouse model, the drug showed three times more anti-cancer efficacy than ARV-110.
Orum Therapeutics is proving its R&D capacity by successfully out-licensing TPD platform to global pharmaceutical companies.
In July last year, the company signed an out-licensing agreement with the U.S.-based biotechnology company Vertex Pharmaceuticals for its TPD.
Vertex Pharmaceuticals is a company that developed a CRISPR/Cas9 gene-edited therapy, exa-cel.
Utilizing Orum Therapeutics' Dual-Precision TPD (TPD2), Vertex Pharmaceuticals plans to discover 'conditioning agents,' which are used to wash bone marrow before injecting patients with gene editing products.
TPD, provided by Orum Therapeutics, is a technology that specifically degrades target proteins.
Daewoong Pharmaceutical and Samjin Pharmaceutical have signed a strategic business agreement with the Korean TPD discovery company Pin Therapeutics to develop novel drugs.
Pin Therapeutics is a novel drug discovery company established in 2017 that conducts global R&D by collaborating with 'PinUS,' a wholly-owned subsidiary in the United States.
Global pharmaceutical companies proactively conduct novel TPD drugs
AstraZeneca has signed an out-licensing deal with the U.S.-based PineTree Therapeutics for exclusive sales and global rights.
PineTree Therapeutics is a company established in 2019 in Boston, U.S., specializing in the development of novel drugs.
PineTree Therapeutics' EGFR degrader candidate was developed using its proprietary multi-antibody platform, 'AbRaptor.' Based on the research, this candidate demonstrated anti-tumor activity when used in tumors resistant to drugs such as tyrosine kinase inhibitor (TKI).
AstraZeneca has the EGFR drugs Tagrisso and Iressa for the treatment of non-small cell lung cancer (NSCLC).
Utilizing the TPD technology, the company plans to develop follow-up drugs for Tagrisso and Iressa.
Global pharmaceutical companies like Pfizer and Novartis have invested over KRW 1 trillion in Arvinas' TPD pipeline development.
The U.S.-based company Arvinas is a leader in the TPD field.
Arvinas' platform PROTAC was previously referred to as TPD technology.
The company is the only company in the world to own a proprietary novel TPD candidate that has entered the Phase 2 trial.
In 2021, Arvinas signed an out-licensing deal with Pfizer for its novel TPD drug, 'ARV-471,' for breast cancer treatment.
It also transferred androgen receptor degrader 'ARV-766' to Novartis.
With the agreement, Novartis will be responsible for the global clinical trial development and commercialization of ARV-766.
Last year, Arvinas announced ARV-766's interim data from dose escalation research.
Based on the clinical results, the drug demonstrated a 50% reduction of prostate-specific antibody (PSA) in 41% of patients with mutations in the ligand-binding domain in the androgen receptor.
Novartis will aim to commercialize ARV-766 as a prostate cancer treatment and enter the later stage of clinical trials.
Additionally, various global companies like Novo Nordisk, Astellas, and Eli Lilly have jumped into this field by investing in companies developing novel TPD drugs.
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.










