- LOGIN
- MemberShip
- 2025-12-22 09:29:52
- 4 of 5 listed pharma companies expand investments
- by Chon, Seung-Hyun | translator Alice Kang | 2024-11-26 05:54:30
Pharmaceutical companies have significantly expanded their research and development (R&D) investments to discover their next item.
Five out of five major pharma and biotech companies increased their R&D spending this year compared to the previous year.
The top pharmaceutical companies in terms of sales have been investing heavily in R&D to develop new drugs.
Yuhan Crop, Celltrion, and Dong-A ST showed a significant increase in R&D expenditures.
According to the Financial Supervisory Service on the 25th, the cumulative R&D investments of 20 major pharmaceutical and biotech companies totaled to KRW 1.867 trillion in Q3, up 14.5% from the KRW 1.6387 trillion in the same period last year.
The data was compiled from 20 listed pharmaceutical companies with the highest sales of main drug products.
Ildong Pharmaceutical, which spun off its R&D subsidiary last year, was not included in the survey.
Sixteen of the 20 major pharmaceutical companies increased their R&D investment this year compared to the same period last year.
Celltrion, Samsung Biologics, Yuhan Corp, Daewoong Pharmaceutical, Hanmi Pharmaceutical, SK Biopharmaceuticals, Chong Kun Dang, Dong-A ST, HK Inno.N, JW Pharmaceutical, Boryung Pharmaceutical, Daewon Pharm, Huons, Dongkook Pharmaceutical, Dongwha Pharmaceutical, and Celltrion Pharmaceutical showed an increase in R&D expenditures through the third quarter compared to last year.
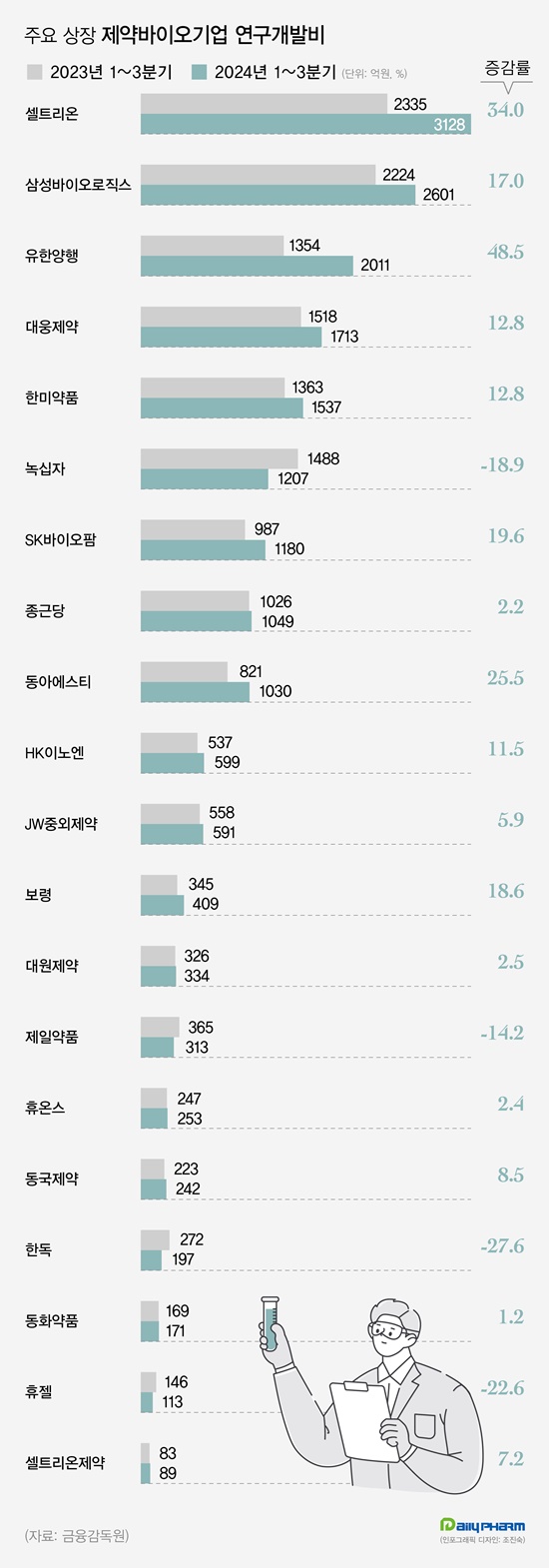
The company invested KRW 201.1 billion in R&D through the third quarter, up 48.5% from KRW 135.4 billion it had invested in the same period last year. The increase was largely due to the reallocation of technology fees it accrued from its anti-cancer drug Leclaza.
In August, the U.S.
Food and Drug Administration (FDA) approved Leclaza in combination with Rybrevant for the first-line treatment of adult patients with locally advanced or metastatic non-small cell lung cancer (NSCLC) with a confirmed epidermal growth factor receptor (EGFR) exon 19 deletion or exon 21 L858R substitution mutation.
Yuhan Corp received a USD 60 million technology fee from Janssen Biotech for the FDA approval of Leclaza.
Of the total Leclaza royalties received, 40% was paid to the original developer, Oscotec.
In 2016, the company acquired the preclinical development rights to Leclaza from Oscotec and its subsidiary Genosco.
The technology fees redistributed to Oscotec were recognized as R&D expenses.
This significantly increased the company’s R&D expenses as Yuhan paid more than KRW 30 billion to Oscotec out of the royalties it had received for Leclaza.
In the first half of the year, Yuhan’s R&D expenses were high due to the introduction of promising technologies from bioventures.
In March, the company paid KRW 6 billion to acquire the technology of SOS1-inhibiting anti-cancer drug candidates from Cyrus Therapeutics and Kanap Therapeutics.
In the second quarter, the bank paid KRW 3 billion in technology fees to a biotech company, J Ints Bio.
Celltrion reported cumulative R&D expenses of KRW 312.8 billion in the third quarter, up 34.0% YoY.
Celltrion has received approval for 2 biosimilars in Europe so far this year.
In May, the company received marketing authorization from the European Commission for its first biosimilar of Xolair, Omlyclo.
Xolair is an antibody-based biological drug used to treat allergic asthma, chronic rhinosinusitis with nasal polyps, and chronic idiopathic urticaria. In August, the company received approval for SteQeyma, a biosimilar to the autoimmune disease treatment Stelara, received European marketing authorization.
Stella Stelara is a Janssen-developed autoimmune disease treatment for plaque psoriasis, psoriatic arthritis, Crohn's disease, and ulcerative colitis.
Celltrion has acquired 8 and 6 approvals in Europe and the U.S., respectively.
Celltrion is developing follow-on biosimilars for Keytruda, Prolia, Actemra, Cosentyx, and Ocrevus. Dong-A S&T's R&D expenditure through the third quarter was KRW 103 billion, up 25.5% YoY.
Its clinical expenses for new drug development increased significantly.
DA-4505, an immuno-oncology drug, was approved for Phase I/IIa clinical trials in Korea in November last year.
DA-4505 showed improved tumor suppression through preclinical studies compared to AhR antagonists being developed by global pharmaceutical companies.
A Phase III clinical trial for DA-8010, a treatment for overactive bladder, was completed in Korea in May.
However, DA-8010 did not show a statistically significant difference. In October, Dong-A ST’s Stelara biosimilar Imuldosa received final approval from the FDA, marking the company's entry into the U.S.
market.
The company passed the U.S.
market gateway 11 years after starting the development of Imuldosa in 2013.
SK Biopharmaceuticals, Boryung, Samsung Biologics, Daewoong Pharmaceuticals, Hanmi Pharmaceuticals, and HK Inno.N have expanded their R&D expenditures by more than 10% YoY through the third quarter of this year. The increase in R&D expenditures was larger among pharmaceutical companies with larger sales.
Samsung Biologics, Celltrion, Yuhan Corp, GC Biopharma, Chong Kun Dang, Hanmi Pharmaceutical, Daewoong Pharmaceutical, Boryung Pharmaceutical, and HK Inno.N have invested KRW 1.49 trillion in R&D this year, up 16.8% from the previous year.
Of the top 10 companies by revenue, nine, except for GC Biopharma, increased their investment from last year.
It is analyzed that large pharmaceutical companies, which have accumulated experience in developing new drugs and are actively seeking to expand globally, have been actively investing in R&D to discover new items.
SK Biopharmaceuticals had the highest R&D investment-to-sales ratio, at 30.7%.
Dong-A ST and Daewoong Pharmaceutical followed with 19.9% and 18.3%, respectively, while Hanmi Pharmaceutical, Yuhan Corp, Celltrion, and JW Pharmaceutical also invested more than 10% of their sales in R&D.
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.










