- LOGIN
- MemberShip
- 2025-12-22 12:52:34
- Auto part company confirms potential of novel antibody drug
- by Son, Hyung Min | translator Hong, Ji Yeon | 2024-12-09 05:46:59
Kumho HT's candidate product for cancer immunotherapy demonstrated drug tolerance and effectiveness in human-subject clinical trials.
At the European Society for Medical Oncology (ESMO) Asia Congress 2024, held for three days from December 6, the Phase 1 clinical trial result of Kumho HT's DNP-002, a new anticancer drug, has been unveiled.
Kumho HT anticipates collaboration with global pharmaceutical companies based on the results from the Phase 1 clinical trial.
In 2021, Kumho HT, an automotive electronics company, acquired the antibody drug developer DiNonA, officially making an entry into the biotechnology sector.
The company's largest shareholder, S-MAC, aims to secure a new revenue stream through this acquisition, diversifying its business portfolio beyond electronic components and materials.
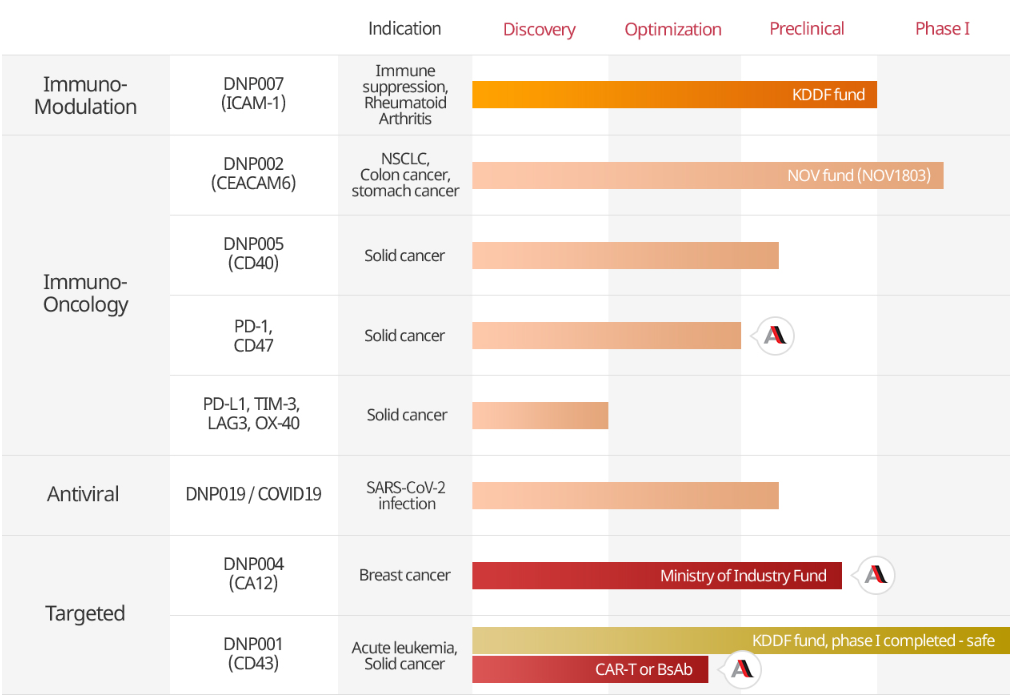
Based on DiNonA's antibody drug development platform, Kumho HT is developing anticancer agents, immune modulators, and COVID-19 therapies.
Cancer immunotherapy with a novel mechanism demonstrates results in the Phase 1 trial

The current result has been presented in about four years after the approval of the Investigational New Drug Application (IND) in South Korea in 2020.
DNP-002 targets 'CEACAM6,' a protein overexpressed in cancer cells and myeloid-derived suppressor cells (MDSC).
By targeting both tumor and MDSC, it re-activates the patient's immune system.
CEACAM is a novel target protein known to selectively target regulatory T cells expressed within tumors without affecting T cells.
Cancer immunotherapy and antibody-drug conjugates (ADCs) targeting CEACAM 1, 5, and 6 are undergoing clinical trials for major solid tumors such as gastric and esophageal cancers.
The clinical trial involved 36 patients with solid tumors registered at Asan Medical Center in Seoul and the National Cancer Center.
The study aimed to evaluate the efficacy, safety, and tolerability of DNP-002.
Participants were 18 years or older, had a prior treatment history, and had an Eastern Cooperative Oncology Group Performance Status (ECOG PS) of 1 or lower.
Higher ECOG scores indicate more severe symptoms.
The cohort was divided into four groups based on patient characteristics.
Clinical results showed that 1 out of 12 patients who could be evaluated for tumor assessment had a partial response (PR) of over 30% reduction in cancer cells.
Seven patients were confirmed to have a stable disease (SD) with maintained tumor size.
Patients with esophageal cancer registered to another cohort group showed a total of 69% reduction in tumor size compared to the baseline of administration and maintained PR for 30 weeks.
Researchers stated, "0.03mg/kg of DNP002 treatment demonstrated a partial response and drug tolerance.
We are conducting a study to determine the maximum drug dosage." Automotive electronics company acquires a novel drug developer, making an entry into the biotech industry An automotive electronics company, Kumho HT, began generating results in developing cancer immunotherapy in 2021.
At that time, Kumho HT's largest shareholder, S-MAC's Chief Executive Officer & Director Kyung-Suk Cho, acquired DiNonA, a company developing novel antibody drugs.
Cho established a corporate governance structure spanning Ohsung Advanced Materials-S-MAC-Kumho HT-DiNonA-Hwail Pharmaceutical through 'East Burgundy,' a management consulting firm wholly owned by Cho.
Cho identified biotechnology as a future cash cow, transitioning from a business structure focused on automotive parts and materials.
DiNonA, acquired by Kumho HT, specializes in antibody-based drug development and is currently conducting clinical trials for three anticancer drug candidates.
One of these is 'DNP-002,' for which the Phase 1 clinical trial results were recently disclosed.
Additionally, the company is conducting the clinical development of 'DNP-007,' an immunosuppressant candidate that received IND approval for Phase 1 in 2021.
Previously, in 2018, DiNonA also licensed four antibody drug candidates to Aprogen.
Immune tolerance is the process by which immune cells are prevented from attacking one of our cells.
Without proper immune tolerance, autoimmune diseases such as rheumatoid arthritis and multiple sclerosis can occur.
DNP-007 is an antibody therapy known as MD-3, a humanized anti-ICAM-1 antibody that employs a novel mechanism to induce immunosuppression in transplanted organs by modulating dendritic cells.
The company is conducting joint research with Seoul National University Hospital for DNP-007.
According to recently released preclinical study results, monkeys that received continuous administration of DNP-007 maintained normal liver function for over three years, while those treated with conventional immunosuppressants survived less than three months.
The company aims to develop 'DNP-007' as an alternative to calcineurin inhibitors, which are known to cause severe side effects such as diabetes, neurotoxicity, renal dysfunction, and alopecia.
Based on the antibody drug candidates acquired through DiNonA, Kumho HT is exploring the potential for developing a wide range of antibody-based therapies in addition to the field of oncology.
The company has completed Phase 1 clinical trials for its leukemia antibody therapy 'DNP-001.' An ongoing study is also being conducted for the COVID-19 treatment 'DNP-019' and the atopic dermatitis therapy 'KHT-2031' for pets.
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.










