- LOGIN
- MemberShip
- 2025-12-21 21:56:44
- Companies produced KRW 66.7B finished drugs a year
- by Chon, Seung-Hyun | translator Alice Kang | 2025-01-24 05:52:15
The average production performance of pharmaceutical companies has been gradually increasing.
As the scale of the pharmaceutical industry grew, the average production value of both finished drugs and raw materials also continued to grow.
For both finished drugs and raw materials, the share of small companies with annual production of less than KRW 10 billion was overwhelmingly large.
According to the MFDS’s '2024 Food and Drug Statistical Yearbook,' 399 pharmaceutical companies produced a total of KRW 25.57 trillion worth of finished drugs in 2023.
On average, each pharmaceutical company produced KRW 64.1 billion’s worth of finished drugs.
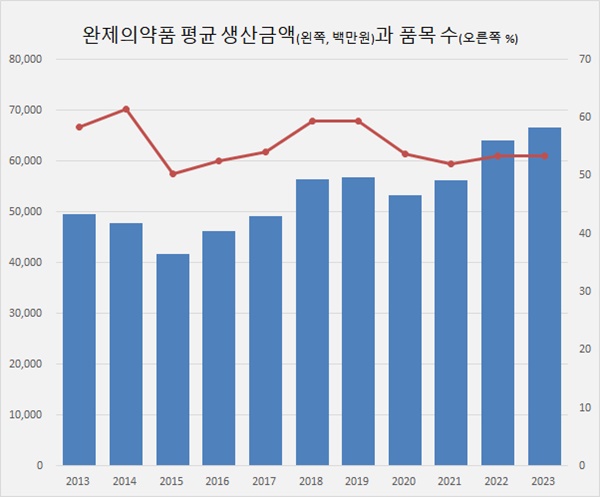
As the pharmaceutical industry continues to grow, the average production value of pharmaceutical companies has also expanded.
In 2023, the number of finished drug items produced by each pharmaceutical company was 53.4.
This is the 8 less in nine years since 2014 when it reached 61.4.
The production value per finished drug item in 2023 was KRW 1.249 billion, up 4.1% year-on-year from KRW 1.249 billion in 2022.
The average production value of finished pharmaceutical products has increased by 60.6% over nine years, from KRW 777.93 million in 2014.
This means that pharmaceutical companies are restructuring their products, reducing the number of finished drug items they own, and becoming larger companies with higher production values per item.
However, the pharmaceutical industry as a whole was dominated by small companies.
Of the 403 producers of finished pharmaceutical products in 2023, 204 companies with a production value of less than KRW 10 billion accounted for 50.6%.
This means that more than one in two pharmaceutical companies are SMEs with annual production of less than KRW 10 billion.
There were 126 manufacturers with less than KRW 1 billion in finished drug production.
This means that one out of every three manufacturers is a small business with less than KRW 1 billion in annual production.
The number of companies producing less than KRW 1 billion in finished pharmaceuticals has tripled in 10 years, from just 45 in 2013.
In 2023, 73 companies produced more than KRW 100 billion in finished pharmaceutical products, an increase of 8 companies from the previous year.
Compared to 38 companies in 2013, the number of companies with a production value of KRW 100 billion or more increased by 92.1%.
The number of companies producing more than KRW 500 billion in finished pharmaceuticals has tripled in 10 years, from 4 in 2013 to 12 in 2023.
Raw material drug companies are also scaling up their businesses, but the share of small companies is still large.
In 2023, 296 raw material drug makers produced a total of KRW 3.768 trillion.
Each raw material drug company produced an average of KRW 12.7 billion.
The average production value of raw material drug companies has more than doubled in 10 years from KRW 5.9 billion in 2013.
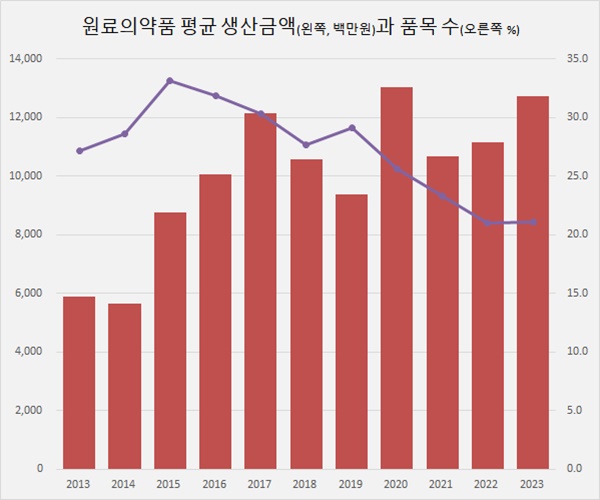
Similar to finished pharmaceuticals, raw material drug manufacturers are also analyzed to improve their quality by reducing the number of products they handle while increasing the average production scale.
In 2023, 239 of the 296 producers of raw material pharmaceuticals made under KRW 10 billion, accounting for 80.7%.
The number of companies producing less than KRW 1 billion was 137, or 46.4%.
Almost half of the companies produced less than KRW 1 billion in annual output.
The number of companies producing less than KRW 1 billion in raw materials decreased by 106 over the 10 years from 243 in 2013.
The number of companies producing more than KRW 100 billion in raw materials more than doubled in 10 years, from just three in 2013 to seven in 2023.
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.










